User:Gg207
1.21 The Hydrogenation of a Cyclopentadiene Dimer
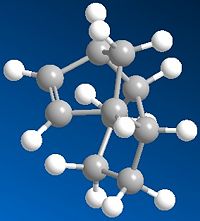
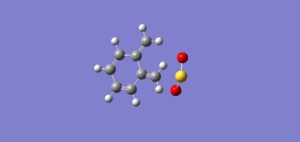
Molecule 1 and 2 were drawn into ChemBio Draw 3D, their geometries were modified using MM2 and their energies were recorded. The energy for product 1 was noted to be 129.1kJ/mol (the program calculated the energy to be 31kcal/mol) and the energy for product 2 was found to be 142.3kJ/mol (the program calculated the energy to be 34kcal/mol)
 |
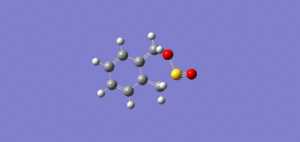 |
From this data it can be seen that product 1 is lower in energy and is therefore more stable than product 2. However it is product 2,in the endo form, that is the dominant product in this Diels Alder Cycloaddition. Product 2 is the kinetic product and although it is at a higher energy its transition state is at a lower energy and this is why the endo product is formed more favourably. The reason for this can be explained using the endo addition rule. The orbitals at the back of the diene are orientated in such a way so that bonding MOs can be formed between it and the orbitals on the dienoplhile. Although no new bonding orbitals are formed this favourable overlap ensures that the endo product is the fastest formed and thus explains why it is the dominant product. [1]
MM2 is not equipped to calculate orbital interactions, and so looks at the thermodynamic model. The endo orientation is thermodynamically less stable due to steric clash. However the activation energy barrier that needs to be overcome is between the reactants and transition state and so a thermodynamic outlook is not always the best available way to predict the outcome of a reaction.
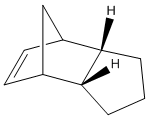
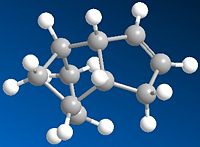
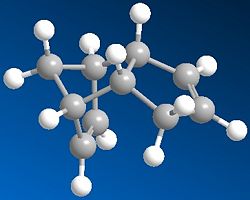
If we are to look at the two values for the bend of the molecules it can be noted that product for has a much lower bend than that of product 3. In the ChemBio 3D program the bend tells us the “energy associated with deforming bond angles away from their optimum values”[2] therefore the one at the lowest energy is the most stable so looking at the bend shows that product 4 is the most stable isomer. This agrees with the fact that the 6 membered ring has been hydrogenated over the 5 membered ring, so that the most stable bond angles for the structure can be achieved.
Torsion is another factor that needs to be looked at when decided which of these two products is the most stable. The torsion value for product 3 is 10.9 and for product 4 it is 12.5.
References
1.22 Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
Pentahelicene |
Molecule 5 was drawn without the MeMgI component and then its geometry was minimised using MM2.It was attempted to find the lowest energy structure for the molecule, but as energy was not as important as other factors in this exercise it was decided to focus on the geometry of the carbonyl group. The reaction shows that the methyl group has been added on the top face of the molecule. The carbonyl group of the minimised reactant is also on the top face.To ensure that the correct geometry of the carbonyl group was obtained the structure was manually manipulated in ChemBio 3D and then minimised. This was repeated several times until it was felt that the correct geometry and orientaion fo the carbonly group was found. This is shown by measuring its dihedral angle which was found to be 12° as calculated by ChemBio 3D.[1]
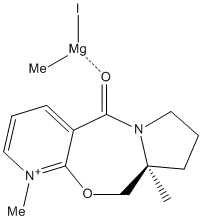 The reason for the particular stereochemistry of 6 is because of chelation control. The magnesium is coordinated to the carbonyl group and thus changes the conformation. Rather than placing the methyl group perpendicular to the carbonyl group, instead the chelation of Mg ensure that Me can lie as close to the carbonyl as possible. This means that the Me group has no choice but to add onto the same face as the carbonyl group. If the carbonyl group was situated below the ring then this is where the Me would have been placed. The chelation to the carbonyl, and the position of this functional group of the molecule decides the regioselectivity of the product. [2] |
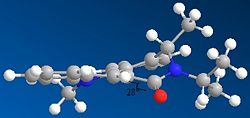
The same method was used to minimise molecule 7. Here the carbonyl is situated below the ring.Again the structure was manipulated and minimised several times in order to confirm at the correct geometry of the coarbonly group and that in this case the carbonyl group is to be found below the plane of the ring. The reactants do not contain any metals so in this instance chelation control does not occur. The stereochemistry can be explained by the repulsion of the lone pairs on the carbonyl with that on the nitrogen. In the case of 7 the carbonyl group is situated below the ring and this can be seen by its dihedral angle which is at 28°.
What is missing from this model is chelation control. Aspects of module 3 would have been useful for this question as it would have been beneficial to analyse the transition state of the reaction of 5 to 6, so to see which face the Me would add to.
References
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
- ↑ Organic Chemistry; Clayden, Greeves, Warren and Wothers p 892-4, p1063-6
1.23 Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
The first Jmol image shows 10, the second shows 11.
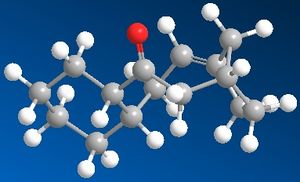
Molecule 10 was drawn out and minimised. At first and energy of 234kJ/mol was calculated, but to ensure that the optimum energy was achieved the structure was manually manipulated using the computer program and minimised several times. The manual manipulation meant that the structure could be better understood, and so deciding what geometry to chagne becoame an easier task. Eventually energy of 172kJ/mol was achieved. In product 10 the carbonyl group is pointing up in respect to the bridgehead.
The same was done for molecule 11, in this conformer the carbonyl group is pointing down in respect to the bridgehead.The two images show two different conformations of the same molecule. The first image had energy of 188kJ/mol, but again this was manually manipulated until a much lower energy form of the structure was found and this was at 167kJ/mol.
 |
 |
This shows that molecule 11 is in fact the most stable. However the energy difference is not that great between the two. These two molecules cannot interconvert into one another so therefore a small energy difference will suffice as evidence which is the more stable of the two.
The double bond reacts slowly due to its position in the ring, and the fact that the molecules are hyperstable olefins. Both of these molecules have a double bond next to the bridgehead. This is allowed in accordance to Bredt’s Rule, as the ring system is large enough to contain a double bond in this position. This double bond here is actually hyper stable, that is to say that the alkane is more strained than the double bond.
Aspects of the structure that could be futher optimised is the six membered ring. This structure will obviously prefer to be in the chair conformation, so the six membered ring can be concentrated on and its geometry improved. This could lower the energy of the molecule and thus stabilise it.
1.24 Modelling Using Semi-empirical Molecular Orbital Theory
Part 1
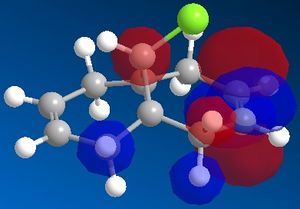
By looking at the HOMO it is hard to see which alkene is the most nucleophillic. The C=C bond with the most build up of electron density is the most nucleophillic one. This should be apparent from the HOMO, however in this case more information about this can be gathered from the HOMO-1. As it can be seen from the picture, the majority of the electron density is situated around the anti double bond, the one nearest to the Chloride.[1] DOI:10.1039/P29920000447
|
|
shown above is one of the C=C stretches on the diene and the C-Cl vibration on the diene at 770cm-1
 |
 |
 |
 |
 |
Part 2
The C-Cl and C=C stretches for the diene are as follows: C-Cl is at 770cm-1, endo double bond is at 1737.10cm-1 and exo double bond is at 1757.36cm-1. For the molecule without the anti double bond the C-Cl stretch is at 775cm-1 and the C=C bond is at 1758cm-1.
It can be seen that the C-Cl stretch is larger for the molecule that contains only one double bond. As IR measures the vibrations of the molecule that larger the frequency the stronger the bond. Therefore it can be concluded that the C-Cl in the diene is a weaker bond. The reason for this can be found by looking at the molecular orbital’s for this molecule.
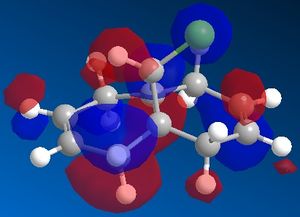
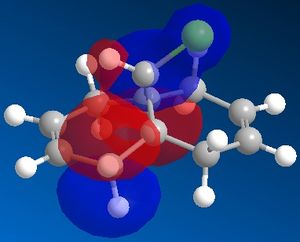
By examining the LUMO+1 and the HOMO-1 of the diene an unfavourable overlap can be seen between the sigma antibonding orbital of the C-Cl bond and the pi bonding orbital of the C=C bond. The exo pi orbitals are occupied donatinf electron density.
On the hydrogenated product there is no pi orbital present to interact with the C-Cl antibonding sigma orbital and so therefore this bond is stronger.
Mini Project-Differentiatin between the Cis and Trans Isomers of an allyl substituted Piperidone
The aim of this project is to see if comutational methods are just as efficient as classical spectroscopy in differentaiting between the two stereoisomers of an allyl substituted piperidone.
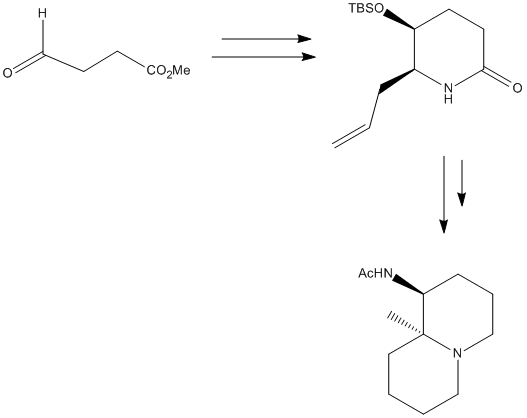 The overall reaction is shown in the image, however the moecule to be analysed is the allyl substituted piperidone in the intermediate step. [2] The R and S cyanohydrins were synthesised first from the semialdehyde. The equilibrium can be forced over to the side of the cyanohydrins by using a two phase system. The synthesis favours the S enantiomer, which was a favourable coincidence as the R enantiomer was difficult to scale up resulting in a loss of yield. The synthesis is described below. This product is made from the (S)-configured cyanohydrins, which was made using the starting aldehyde and reacting it with HbHnl and MTBE buffer at pH 4.5, then with TBSCl. The cyanohydrins was dissolved in MeOH . Pd was dadded and the hydrogen was bubbled through the solution for 40 mins, then filtered over Celite and concentrated to give a crude white solid. This was then repeated 8 times so that enough of the crude product could be collected. This crude product was then dissolved in acetic acid and cooled to 0°C. NaNo2 was added and after 15 mins the cooling bath was removed and the mixture was stirred overnight. NaHCO3 and EtOAc was added and then the mixture was neutralised using Na2CO3. The mixture was then extracted with EtOAc and brine, then this was dried over Na2SO4 and concentrated to give a yellow oil. This was then evaporated twice in toluene and dissolved in dichloromethane. More steps were done to purify the product and the product was formed in a ratio of 4.2:1 in favour of the cis isomer. The mechansim for this is shown here. 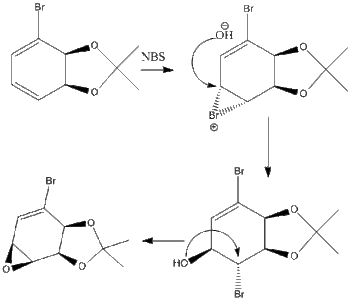
The cis and the trans product were drawn out into ChemBio 3D and were optimised using MM2. The TBSO group was manually manipulated so as to get the lowest energy form of the molecule. The alkyl groups on the TBSO were rotated by 60°so that the lowest energy conformation of the molecule could be found. When the dihedral angles were at 60°, so in the gauche formation this was when the molecule was at its lowest energy form. The same was found for the trans isomer. The geometry optimisation was sent to SCAN, and once this was returned the optimised .gif file for the cis and trans isomer was sent to SCAN to obtain NMR and IR data. This data was then compared to the literature and the results are as follows for the cis and trans isomers.
The dihedral angles between the hydrogen’s of the stereocentres on the cis and trans isomers were then calculated to see if the two isomers can be differentiated between using coupling constants and 1H NMR. For the cis isomer the dihedral angle was found to be 138° and for the trans isomer the dihedral angle was found to be 47.6°. When placed in the Karplus equation this gave a 3J coupling constant of 4.09 and 2.69 retrospectively. For the cis isomer the chemical shift of the hydrogen nearest to the nitrogen should be around 3.5pp and for the hydrogen nearest the oxygen it should be around 4.5ppm. The literature reposts a double triplet at 3.36ppm with a J value of 3.4. This agrees with the findings from the dihedral angles and Karplus calculations. However the literature does not report any J coupling values for the trans isomer and therefore 1H NMR is not the best method to use to differentiate between the two isomers. [3] However the 13C NMR can also detect some differences between the cis and the trans isomer. The carbons on the TBSO group in the trans isomer are at a higher ppm than that of the cis isomer. These carbons are labelled on the diagram and can be seen in the table. Also the literature data was higher than the data gathered form the optimised geometry. The IR data was obtained for the 2 isomers by submitting the optimised file to SCAN and the opening the checkpoint file in Gausview and looking at the vibrations. For the cis isomer the IR results are as follows, with the data from the literature and with what vibration the frequency corresponds to( all IR data is civen in wavenumbers with the unit cm-1):
Other differences are the frequency at which the cis and trans hydrogen’s vibrated at. The cis hydrogen’s had a frequency of 2983cm-1 and the trans hydrogen’s vibrated at a frequency of 2988cm-1. The difference may only be slight but it still does vary for the two different isomers.
|