User:Gck12
Borane
Basis set 3-21G
Optimisation file here
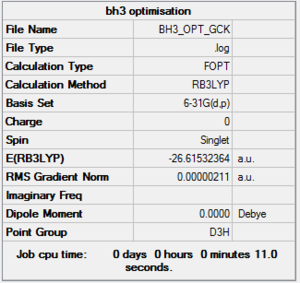
Basis set 6-31G(d,p)
Optimisation file here
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|
|
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000017 0.001800 YES
RMS Displacement 0.000011 0.001200 YES
Predicted change in Energy=-1.021829D-10
Optimization completed.
-- Stationary point found.
|
|
GaBr3
Basis set B3LYP/LANL2DZ
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|

|
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000003 0.001800 YES
RMS Displacement 0.000002 0.001200 YES
Predicted change in Energy=-1.307745D-12
Optimization completed.
-- Stationary point found.
|
|
BBr3
Basis set B3LYP/6-31G(d,p)LANL2DZ
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|

|
Item Value Threshold Converged?
Maximum Force 0.000008 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000036 0.001800 YES
RMS Displacement 0.000024 0.001200 YES
Predicted change in Energy=-4.086270D-10
Optimization completed.
-- Stationary point found.
|
|
| Parameter | BH3 | BBr3 | GaBr3 |
|---|---|---|---|
| r(E-X) | 1.19 | 2.02 | 2.39 |
| θ(X-E-X) | 120.0 | 120.0 | 120.0 |
Answers to questions
Reference for covalent radii data are taken from: Beatriz Cordero, Verónica Gómez, Ana E. Platero-Prats, Marc Revés, Jorge Echeverría, Eduard Cremades, Flavia Barragán and Santiago Alvarez (2008). "Covalent radii revisited". Dalton Trans. (21): 2832–2838
Comparison of BH3, BBr3 and GaBr3
All compounds are trigonal planar species and are electron deficient. The bond length will be expected to increase as you go from BH3, BBr3 to GaBr3. This is due to the increasing size of the atoms, which is what is observed. Using a purely covalent bonding model, the bond length will be the sum of the covalent radii between the central atom and the substituent atom. The predicted bond length of B-H, assuming a pure covalent bond, is 115pm which is very close to the calculated bond length of 119pm. The proximity to the theoretical bond length shows that the bonds in BH3 are almost ideal covalent bonds.
As we change the substituent from H to Br, the bond length will increase. The predicted bond length in BBr3 is 204pm, which is longer than the calculated bond length of 193pm. Boron is an electron deficient atom and will therefore readily accept electrons and act as a Lewis acids. The electron density to relieve the electron deficiency in boron could come from an adduct or from surrounding atoms of the compound. In this case, bromine has a lone pair which is not present in a hydrogen atom. Therefore the bromine atom can donate a lone pair of electron into the empty sp2 of Boron; resulting in π stabilisation and an increase in the bond order of BBr3.
On changing the central atom from boron to gallium, the Lewis acidity decreases. Since gallium is lower down the group than boron, the number of orbitals increase as well as the orbitals becoming more diffuse. The mismatch of orbital size between the empty sp2 orbital in gallium and the p orbital with the residing lone pair means there is a poor overlap in orbitals. This results in a very weak π stabilisation effect and does not significantly affect the bond order going from BBr3 to GaBr3. Therefore the predicted bond length of 238pm is almost exactly the same as the calculated value.
What is a bond?
A bond can viewed as the optimal distance between two nuclei and is the minimum point on the Lennard-Jones potential. This is a stationary point on the curve; the second derivative and hence the force of the molecule is equal to zero. This means the attraction between the nuclei and the electrons are in equilibrium to the repulsion of the positive nuclei. The majority of the electron density is shared between the two atoms that makes up the bond. However this definition of a bond does not taken lone pairs and partial bonds into consideration, and only relates to a pure covalent bond.
There are multiple forms of bonding which includes Van de Waals forces, polar, ionic, metallic as well as bonds with characters that are between covalent and ionic. The reasoning behind these bonds are different to that of a covalent bond. Van der Waals forces arises due to dispersion of electron density about the molecule and creates partial charges to interact with other molecules. An ionic bond is formed from the electrostatic force of attraction between oppositely charged ions to form a lattice. For a metallic bond, the cations are held together by a sea of delocalised valence electrons.
The strength of the bond will also vary within each type of bond as well as between the different types of bonds. Ionic bonds will generally be stronger than covalent bonds as ionic bonds can be modelled by point charges and therefore Coulomb’s Law, whereas covalent bonds have an electron cloud around the two nuclei.
The bond strength in covalent molecules will also have a huge variation. For instance, a diatomic N-N triple bond has a bond-dissociation energy of 945kj/mol, with the majority of the electron cloud occupying the space between the two nitrogen nuclei. On the other end of the spectrum, a diatomic fluorine molecule has very low a bond-dissociation energy of 154kj/mol from the repulsion of lone pairs. Even weaker bonds can be found in bridging systems where 3c-2e and 4c-2e bonds are formed through dimers.
Why doesn’t Gaussian always draw bonds?
Gaussian works with pre-set value of bond length for a specific bond. If a bond is weaker than expected due to the circumstances of the molecule, Gaussian will not show the bond as it is lower than the pre-set value. For example a 2c-2e B-H bond in BH3 will fall into the bond length of a B-H single bond, whereas a 3c-2e B-H bond in B2H6 is weaker than the B-H single bond and may not show up in Gaussian.
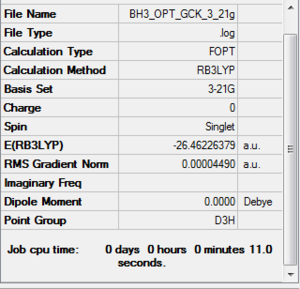
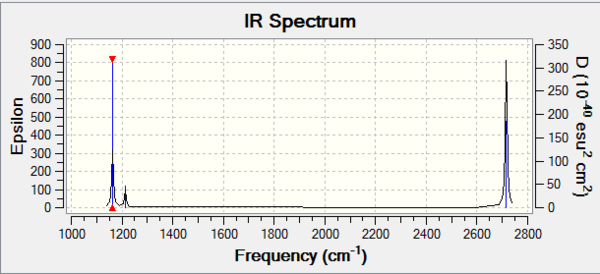
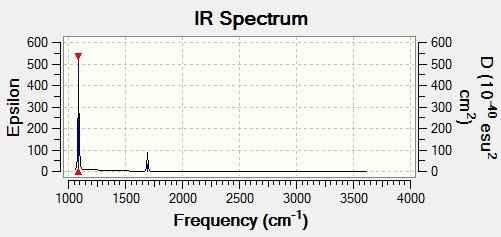
BH3 frequency B3LYP/6-31G(d,p)
Frequency file here
| Summary Data | Low modes |
|---|---|
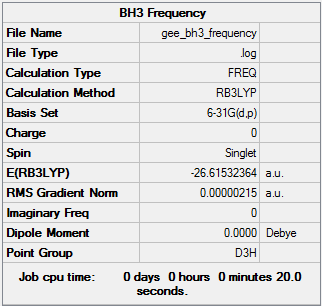 |
Low frequencies --- -11.7227 -11.7149 -6.6070 0.0005 0.0278 0.4278 Low frequencies --- 1162.9743 1213.1388 1213.1390 |
Vibrational spectrum for BH3
| Wavenumber | Intensity | IR active? | Type |
| 1163 | 93 | Yes | Bend |
| 1213 | 14 | Yes | Bend |
| 1213 | 14 | Yes | Bend |
| 2583 | 0 | No | Stretch (Symmetrical) |
| 2716 | 126 | Yes | Stretch (Anti symmetrical) |
| 2716 | 126 | Yes | Stretch (Anti symmetrical) |
Answer to question
Why is there only 5 vibrational modes in the IR spectrum?
For a non-linear molecules the number of vibrational nodes is given by 3N-6, with N being the number of atoms. For BH3 there are 6 vibrational modes, however only 5 are IR active. In order for the vibrational mode to be IR active, there must be a change in dipole moment on vibrational excitation. The wavenumber at 2583cm-1 has a symmetrical stretch hence a change in dipole moment is not induced and is not IR active. This is why there is only 5 peaks in the IR spectrum.
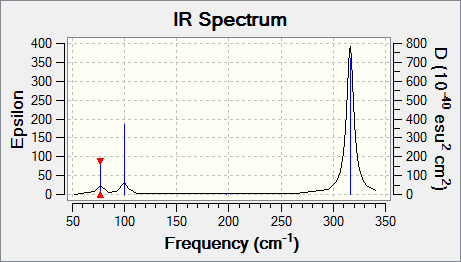
GaBr3 Frequency B3LYP/LANL2DZ
| Summary Data | Low modes |
|---|---|
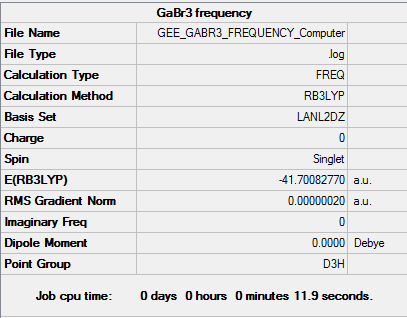 |
Low frequencies --- -1.4878 -0.0015 -0.0002 0.0096 0.6540 0.6540 Low frequencies --- 76.3920 76.3924 99.6767 |
Vibrational spectrum for GaBr3
| Wavenumber | Intensity | IR active? | Type |
| 76 | 3 | Yes | Bend |
| 76 | 3 | Yes | Bend |
| 100 | 9 | Yes | Bend |
| 197 | 0 | No | Stretch (Symmetrical) |
| 316 | 57 | Yes | Stretch |
| 316 | 57 | Yes | Stretch |
Answer to questions
Why must you use the same method and basis set for both the optimisation and frequency analysis calculations?
For the optimisation and frequency calculations to be valid to compare, the same basis set must be used. If the basis set is changed the accuracy level of the calculation will be changed. This can cause the minimum point on the energy curve to shift along the curve, away from the minimum. This will cause a large difference in the energies of the molecules.
What is the purpose of carrying out a frequency analysis?
A frequency analysis is carried out to ensure that your optimisation is not a transition state. The optimisation calculations will find a configuration with an energy gradient of zero. This can either be the minimum point or the maximum point; the transition state. Frequency analysis is the second derivative of the potential energy surface and gives us the curvature of the turning point. When the turning point has positive curvature and hence positive frequencies, the zero-gradient point is at a minimum. The frequency analysis also allows us to locate the IR and Raman modes
What do the "Low frequencies" represent?
Low frequencies represent small distortions from the optimised configuration and arise from rotational and translational modes.
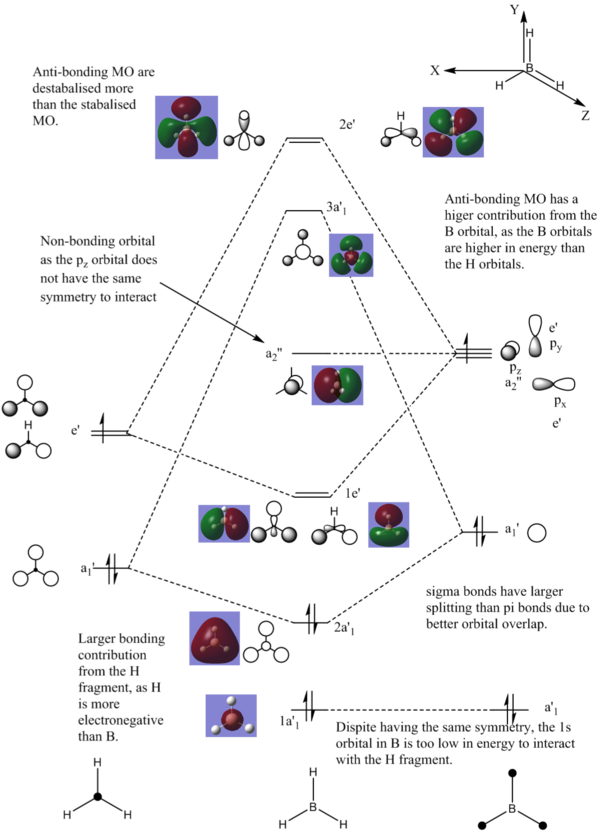
BH3 Molecular Orbital Analysis
Comparing the calculated MO to the LCAO, it can be seen that LCAO is visually a good predictor of the MO. Using LCAO as an approximation for MO is most accurate when used to predict core like orbitals where the calculated and predicted MO are visually similar. For BH3 the energy of B and H are very close which gives a strong overlap of orbitals which makes LCAO a good method of predicting MO in this example. The LCAO becomes less accurate as high energy orbitals are approached as orbitals begin to deform and give poor overlap of atomic orbitals.
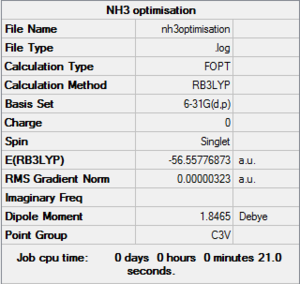
NH3 B3LYP/6-31G(d,p)
Optimisation file here
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|
|
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES Predicted change in Energy=-9.839610D-11 Optimization completed. |
|
Frequency analysis B3LYP/6-31G(d,p)
Frequency file here
| Summary Data | Low modes |
|---|---|
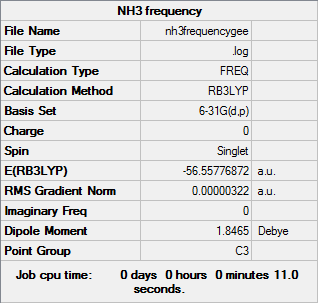 |
Low frequencies --- -0.0137 -0.0031 0.0014 7.0783 8.0932 8.0937 Low frequencies --- 1089.3840 1693.9368 1693.9368 |
| Wavenumber | Intensity | IR active? | Type |
| 1089 | 145 | Yes | Bend |
| 1694 | 14 | Yes | Bend |
| 1694 | 14 | Yes | Bend |
| 3461 | 1 | No | Stretch |
| 3590 | 0 | No | Stretch |
| 3590 | 0 | No | Stretch |
Population analysis B3LYP/6-31G(d,p)
| Atom | Charge |
|---|---|
| N | -1.125 |
| H | 0.375 |
Optimisation of NH3BH3 B3LYP/6-31G(d,p)
Optimisation file here
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|

|
Item Value Threshold Converged?
Maximum Force 0.000122 0.000450 YES
RMS Force 0.000058 0.000300 YES
Maximum Displacement 0.000513 0.001800 YES
RMS Displacement 0.000296 0.001200 YES
Predicted change in Energy=-1.631175D-07
Optimization completed.
-- Stationary point found.
|
|
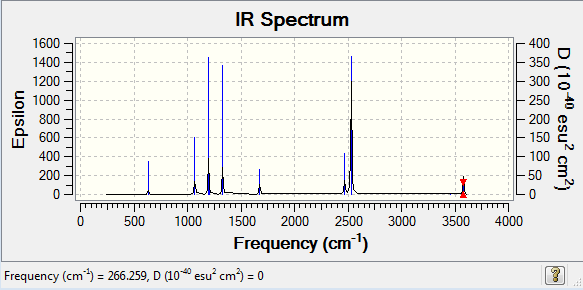
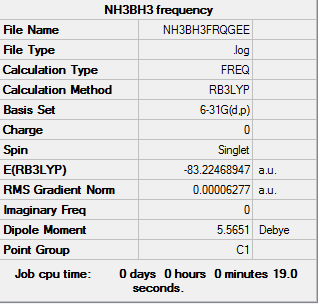
Frequency analysis of NH3BH3
Frequency log file here
| Summary Data | Low modes |
|---|---|
 |
Low frequencies --- -0.0002 0.0006 0.0006 15.2004 18.7944 42.3939 Low frequencies --- 266.2666 632.2878 639.1356 |
Association energies: Ammonia-Borane
E(NH3)= -56.55776873 au
E(BH3)= -26.61532364 au
E(NH3BH3)= -83.22468893 au
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] = -0.05159656 au = -135.47 kj/mol
Analysis of aromatic molecules 6-31G
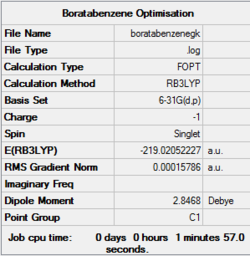
Optimisation of ring molecules
Optimisation file of benzene here
Optimisation file of boratabenzene here
Optimisation file of pyridinium here
Optimisation file of borazine here
| Benzene | Boratabenzene | Pyridinium | Borazine |
|---|---|---|---|
 |
 |
 |

|
Benzene
Item Value Threshold Converged?
Maximum Force 0.000193 0.000450 YES
RMS Force 0.000079 0.000300 YES
Maximum Displacement 0.000830 0.001800 YES
RMS Displacement 0.000294 0.001200 YES
Predicted change in Energy=-4.437902D-07
Optimization completed.
-- Stationary point found.
Boratabenzene
Item Value Threshold Converged?
Maximum Force 0.000159 0.000450 YES
RMS Force 0.000069 0.000300 YES
Maximum Displacement 0.000898 0.001800 YES
RMS Displacement 0.000328 0.001200 YES
Predicted change in Energy=-6.580215D-07
Optimization completed.
-- Stationary point found.
Pyridinium
Item Value Threshold Converged?
Maximum Force 0.000065 0.000450 YES
RMS Force 0.000023 0.000300 YES
Maximum Displacement 0.000832 0.001800 YES
RMS Displacement 0.000177 0.001200 YES
Predicted change in Energy=-7.040945D-08
Optimization completed.
-- Stationary point found.
Borazine
Item Value Threshold Converged?
Maximum Force 0.000085 0.000450 YES
RMS Force 0.000033 0.000300 YES
Maximum Displacement 0.000250 0.001800 YES
RMS Displacement 0.000075 0.001200 YES
Predicted change in Energy=-9.241882D-08
Optimization completed.
-- Stationary point found.
| Benzene | Boratabenzene | Pyridinium | Borazine | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
Frequency analysis of benzene B3LYP/6-31G(d,p)
Frequency log file here
| Summary Data | Low modes |
|---|---|
 |
Low frequencies --- -3.5606 -3.5606 -0.0089 -0.0043 -0.0042 10.0905 Low frequencies --- 413.9582 413.9582 621.1416 |
Frequency analysis of boratabenzene B3LYP/6-31G(d,p)
Frequency log file here
| Summary Data | Low modes |
|---|---|
 |
Low frequencies --- -12.8641 -0.0005 -0.0002 0.0007 15.0587 18.2656 Low frequencies --- 371.3410 404.2589 565.2585 |
Frequency analysis of pyridinium B3LYP/6-31G(d,p)
Frequency log file here
| Summary Data | Low modes |
|---|---|
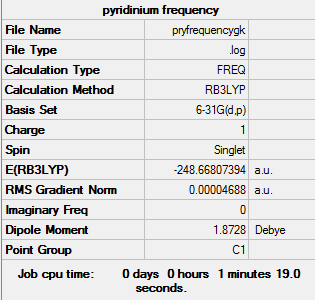 |
Low frequencies --- -7.4767 -0.0013 -0.0010 -0.0010 17.3323 18.6328 Low frequencies --- 392.4468 404.0666 620.4668 |
Frequency analysis of borazine B3LYP/6-31G(d,p)
Frequency log file here
| Summary Data | Low modes |
|---|---|
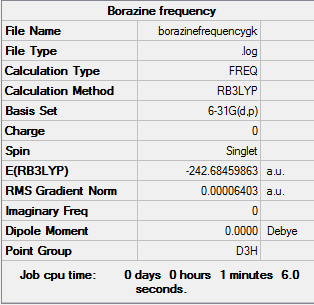 |
Low frequencies --- -2.5283 -0.1112 -0.0039 0.0193 0.6836 0.7915 Low frequencies --- 289.7088 289.7095 404.4210 |
Frequency analysis B3LYP/6-31G(d,p)
Frequency file of benzene here
Frequency file of boratabenzene here
Frequency file of pyridinium here
Frequency file of borazine here
| Benzene | Boratabenzene | Pyridinium | Borazine |
|---|---|---|---|
 |
 |
 |

|
Benzene
Low frequencies --- -3.5606 -3.5606 -0.0089 -0.0043 -0.0042 10.0905 Low frequencies --- 413.9582 413.9582 621.1416
Boratabenzene
Low frequencies --- -12.8641 -0.0005 -0.0002 0.0007 15.0587 18.2656 Low frequencies --- 371.3410 404.2589 565.2585
Pyridinium
Low frequencies --- -7.4767 -0.0013 -0.0010 -0.0010 17.3323 18.6328 Low frequencies --- 392.4468 404.0666 620.4668
Borazine
Low frequencies --- -2.5283 -0.1112 -0.0039 0.0193 0.6836 0.7915 Low frequencies --- 289.7088 289.7095 404.4210
NBO analisys B3LYP/6-31G(d,p)
| Benzene | Boratabenzene | Pyridinium | Borazine |
|---|---|---|---|
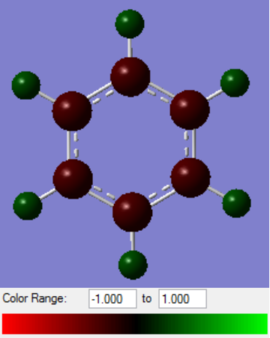 |
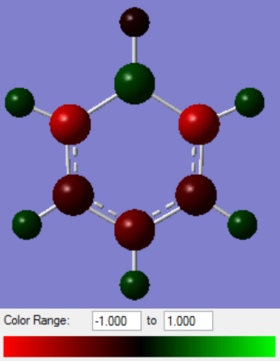 |
 |

|
| Atom | Benzene | Boratabenzene | Pyridinium | Borazine |
|---|---|---|---|---|
| 1 | -0.239 | 0.202 | -0.476 | 0.747 |
| 2 | -0.239 | -0.588 | 0.071 | -1.102 |
| 3 | -0.239 | -0.250 | -0.241 | 0.747 |
| 4 | -0.239 | -0.340 | -0.122 | -1.102 |
| 5 | -0.239 | -0.250 | -0.241 | 0.747 |
| 6 | -0.239 | -0.588 | 0.071 | -1.102 |
| H1 | 0.239 | -0.096 | 0.483 | -0.077 |
| H2 | 0.239 | 0.184 | 0.285 | 0.432 |
| H3 | 0.239 | 0.179 | 0.297 | -0.077 |
| H4 | 0.239 | 0.186 | 0.292 | 0.432 |
| H5 | 0.239 | 0.179 | 0.297 | -0.077 |
| H6 | 0.239 | 0.184 | 0.285 | 0.432 |
Benzene has all carbon atoms in the ring and will have an equal electron density on the molecule. This gives all 6 carbon atoms the same charge of -0.239. The hydrogen atoms are all positive and equal in the magnitude of the charge as all the electron density on hydrogen is directly “pulled” into the each carbon equally.
On changing atom 1 from carbon to boron in boratabenzene, the charge on the hydrogen and carbon change. As boron is less electronegative than hydrogen and carbon, the boron atom will have the most positive charge which is calculated to be 0.202. Carbon atom 2 and 6, next to the boron, will have the most negative charge of -0.588 as carbon has a higher effective nuclear charge than boron and draws electron density away from boron. There is also a negative charge located on the carbon next to the boron, with the negative charge alternating between carbon atom 2 and 6 through the resonance forms. This gives carbon atom 2 and 6 a partial negative charge and is shared between the two carbon atoms. Carbon atom 3 and 5 has a less negative charge than carbon atom 2 and 6 as atom 3 and atom 5 are further away from the boron atom. Therefore the electron density of boron is less accessible for carbon atom 3 and 5. Carbon atom 4 has a more negative charge on than on carbon atom 3 and 5, despite being even further away from the boron atom.
For pyridinium a nitrogen atom is located in atom 1 on the ring and is the most electronegative atom with the highest effective nuclear charge, which gives the most negative charge of -0.476. Carbon atom 2 and 6 of the ring will have its electron density withdrawn by the neighbouring nitrogen atom. As the distance between the carbon and boron atoms increase, the more negative the charge of the carbon. This is because the nuclear charge of nitrogen cannot attract the electron density of the carbon atom enough. Carbon atoms 3, 4 and 5 are negative as the electron density is not sufficiently withdrawn by the nitrogen atom due to distance between atoms. Carbon is also more electronegative than hydrogen, giving a negative charge on carbon.
The ring of borazine alternates between boron and nitrogen atoms. Boron is electropositive and nitrogen is electronegative which gives an alternating pattern of 0.747 and -1.102 charge. As all boron and nitrogen atoms are in the same electronic environment, each atom type will have the same charge and magnitude.
MO of benzene
This MO diagram can be used to show that benzene is an aromatic molecule. The π orbitals show the electron cloud is spread around the ring with no break in the orbital. Furthermore the sigma orbitals are not localised in separate atoms but is connected across the ring, showing the molecule is aromatic. In order for a species to be aromatic, Huckel’s rule must be met. In the case for benzene the 3 π bonding orbital has 6 electrons which obeys the 4n+2 rule, with n=1.
MO comparison
Molecules going from left to right; benzene, boratabenzene, pyridinium, boratabenzene in all pictures.
The HOMO of benzene and pyridinium is constructed from 4 pz orbitals with 2 bonding interactions and 2 anti-bonding interacts and therefore have a similar MO. However the HUMO of pyridinium is lower in energy than benzene, with the energies being -0.47885 au and -0.24691 au for pyrindium and benzene respectively. This is because nitrogen is lower in energy than carbon. Boratabenzene and borazine also have a similar HOMO. The side of the ring ring with more boron atoms appear to have less electron density than the side with carbon for boratabenzene and nitrogen for borazine. This is because boron is the most electropositive atom in the molecules and has the electron density withdrawn by most electronegative atoms. Borazine is also lower in energy than boratabenzene in the HOMO. This is because borazine has 3 nitrogen atoms which is lower in energy than boron and carbon.
In boratabenzene, the boron atom is electropositive relative to the other atoms. This gives carbon a higher electron density around the boron atom. It is the opposite for pyridinium with nitrogen being the most electronegative atom and pulls the electron density towards the nitrogen atom. The contribution is higher on this bonding orbital as nitrogen is lower in energy than carbon.
The LCAO of benzene are all equal in size for each atom which gives an even MO on the 12th orbital. This results in a through space bonding inside and outside the ring. In borazine the majority of the bonding contribution is from the s orbital of boron and hydrogen. This is not the case for the nitrogen atoms due to the mismatch of symmetry that is not like the B-H bond.