User:Ew914
Introduction
The potential energy surface illustrates the energy of a system as a function of two or more coordinates. If there is only one coordinate, it is called a potential energy curve. Two key characteristics of a potential energy surface are called the transition state and the minimum. The transition state is the highest potential energy species along a reaction profile whereas the mimimum is the lowest potential energy species along a reaction profile. Both of these species are turning points on the curve and therefore the gradient is 0.
For this computational lab, gaussian was used along with two electronic structure methods to locate and study the transition states of reactions by calculating the energy and reaction paths of the reactants, products and transitions states of various reactions.
Nf710 (talk) 12:34, 24 February 2017 (UTC) This definition is not detailed enough. A TS is a maximum in alll but one co ordinates and and minimum in the reaction co ordinated.
Exercise 1: Reaction of Butadiene with Ethylene
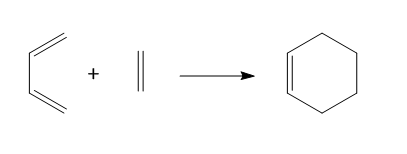
The reaction between butadiene and ethylene is a Diels–Alder reaction or a [4+2] cycloaddition to form cyclohexene. The reaction scheme is shown in Figure 1.
MO Diagram
The MO Diagram for the formation of the butadiene and ethylene transition state is shown in Figure 2. The symmetry of the orbitals is labeled as either symmetric, s or asymmetric, a.
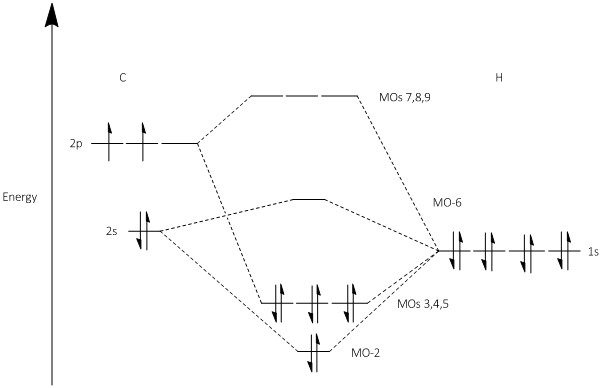
(Fv611 (talk) 11:59, 24 February 2017 (UTC) You have switched the TS antibonding MOs, as the asymmetric one should be the highest in energy)
The MOs for the HOMO and LUMO of butadiane, ethylene and the transition state are shown in Figures 3-8.
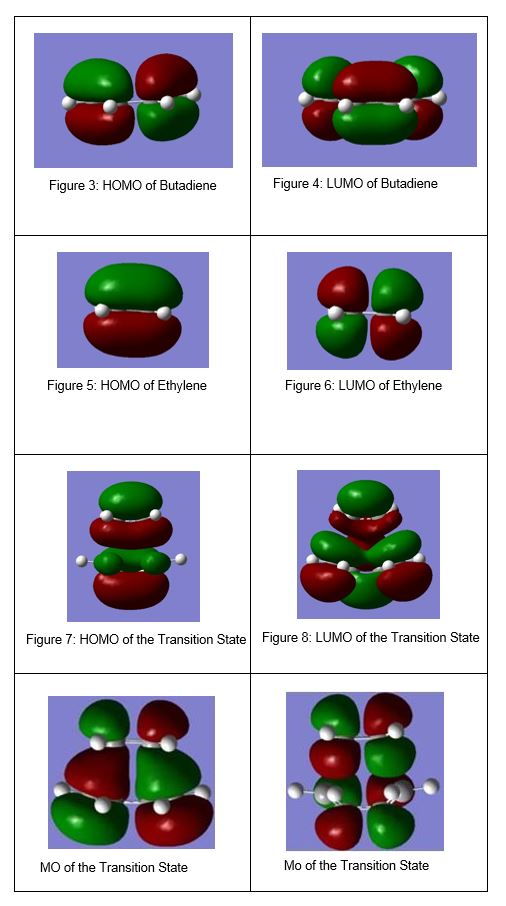
(Fv611 (talk) 11:59, 24 February 2017 (UTC) You should have put a label on the TS MOs to show which one is which.)
A reaction is 'allowed' when the symmetry labels of the orbitals are the same and when the symmetry labels are not the same, the reaction is 'forbidden.' For both a symmetric-symmetric interaction and an asymmetric-asymmetric interaction the overlap integral is non-zero whereas the overlap integral is zero for a symmetric-asymmetric interaction.
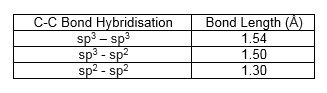
Bond Length Measurements
The measurements of the 4 C-C bond lengths of the reactants and the 6 C-C bond lengths of the transition state and the products is shown in Figure 9. As the reaction progresses, a change in bond length can be observed. The double bonds of the reactants increase in bond length from around 1.3 Å to 1.5 Å, whereas the single bond of the reactants decreased in bond length from around 1.3 Å to 1.5 Å.
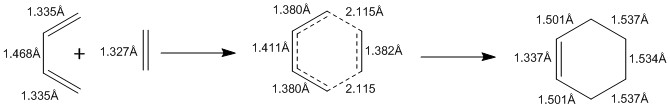
Figure 10 shows the typical C-C bond lengths at different levels of hybridization and the bond lengths from this reaction are in agreement with those bond lengths. The Van der Waals radius of a C atom is 1.70 Å and the partly formed C-C bond length in the transition state is 2.115Å. This confirms that there are bonding interactions between the terminal carbons of the butadiene and the carbons of the ethylene because 2.115 Å is less than 2 x 1.70 Å.
Reaction Path at the Transition State Vibration
Figure 11 shows the reaction path at the transition state which corresponds to the frequency at -526.83 cm-1. This is a synchronous formation of the two bonds.
(Fv611 (talk) 11:59, 24 February 2017 (UTC) This isn't PM6 optimised, but B3LYP.)
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole
Cyclohexadiene and 1,3-dioxole react in a diels-alder reaction as shown in figure 12.
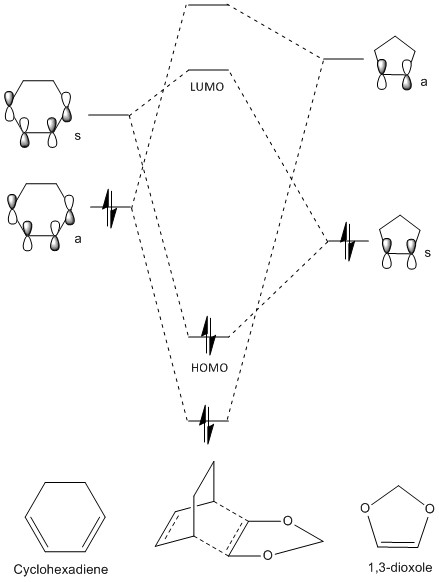
MO Diagram and MOs
A molecular orbital diagram was constructed as shown in figure 13. The oxygen lone pair increases the energy levels of the 1,3-dioxole compared with ethylene. This includes the raising of the HOMO of dioxole which means that it now reacts better with the LUMO of cyclohexadiene because the orbitals are closer in energy.
(Running single point energy calculations on the "reactant" geometry in the IRCs will show that the HOMO of dioxole is actually higher in energy than the HOMO of cyclohexadiene Tam10 (talk) 10:11, 21 February 2017 (UTC))
In diels-alder reactions, normal demand or inverse demand can occur. Normal demand is favoured by an electron poor dienophile and an electron rich diene whereas inverse demand occurs when there is an electron rich dienophile and an electron rich diene which involves the overlap of the HOMO of the dienophile with the unoccupied MO of the diene. The increase in energy of the HOMO and LUMO of the dienophile or 1,3 dioxole due to the oxygen lone pair means that the overlap between the dioxole HOMO and cyclohexadiene LUMO (inverse demand) is better than the overlap between the cyclohexadiene HOMO and dioxole LUMO (normal demand), concluding that this is an inverse demand diels-alder reaction.
The MOs for the HOMO and LUMO of cyclohexadiene, 1,3-dioxole and the exo- and endo- transition states are shown in Figures 14-21.
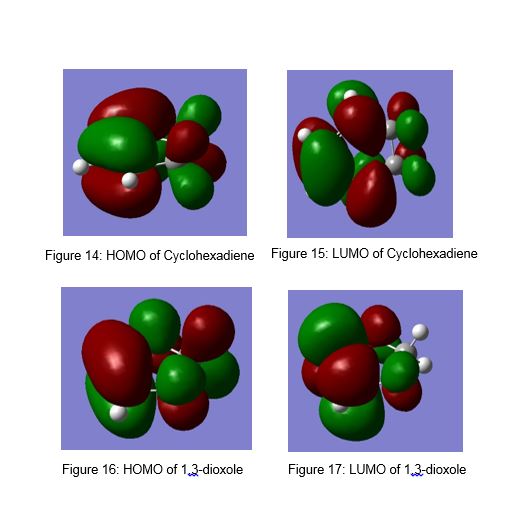
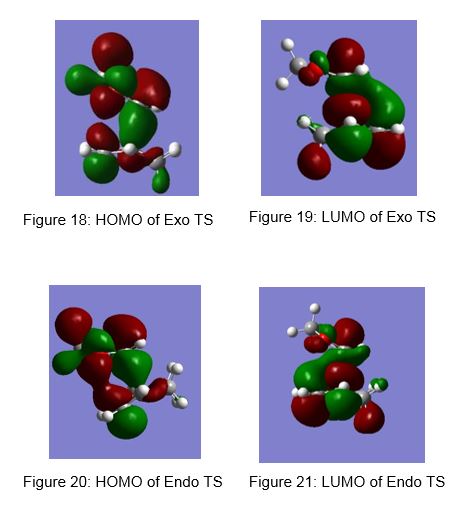
As shown in figure 22, secondary orbital reactions can be seen between the lone pair on the oxgyen and the π* orbital for the endo diels-alder transition state. There is no secondary orbital interaction between the oxygen lone pair and the π* orbital in the exo diels-alder transition state.

(Exo TS looks like the endo TS here. Perhaps this is from a frozen geometry? Tam10 (talk) 10:11, 21 February 2017 (UTC))
The table below shows the obtained activation energies and gibbs free energies for both the endo and exo diels-alder reactions. The activation energy is lower for the endo diels-alder transition state which also shows greater stabilisation than for the exo-diels-alder reaction. With regards to sterics, there is less steric hinderance in the endo transition state than in the exo transition state which aligns with the lower activation energy of the endo transition state. This confirms that the endo diels-alder reaction is both the thermodynamic product and the kinetic product.
| Reactants (KJ/mol) | Transition State (KJ/mol) | Product (KJ/mol) | Ea (KJ/mol) | ΔG (KJ/mol) | |
|---|---|---|---|---|---|
| Exo | -1313533 | -1313364 | -1313601 | 169 | -68 |
| Endo | -1313533 | -1313372 | -1313605 | 161 | -72 |
Nf710 (talk) 12:43, 24 February 2017 (UTC) Correct energies, well done and you have come to the correct conclusion.
Exercise 3: Diels-Alder vs Cheletropic
The reaction between xylene and SO2 can take place via a cheletropic reaction, an endo diels-alder reaction or an exo diels-alder reaction. The reaction scheme is shown in figure 23.
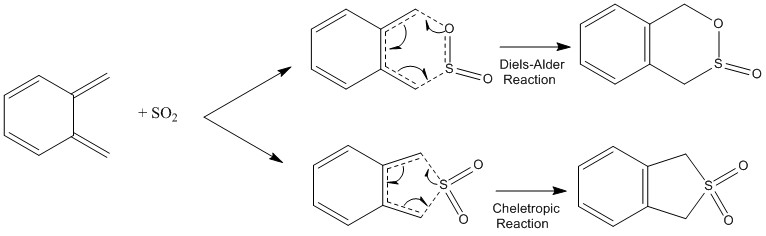
(Don't use half arrows. They imply a single electron Tam10 (talk) 10:11, 21 February 2017 (UTC))
An IRC calculation for each pathway along with the animation is shown in figure 24. Xylene is unstable and very reactive because it contains a large number of double bonds and is not aromatic. Looking at the IRC, a partial double bond is formed in the xylene, then the C-O and C-S sigma bonds are formed and then the partial double bond and SO2 is formed to complete the reaction and produce a stable aromatic product.
| Exo Diels-Alder Reaction |  |
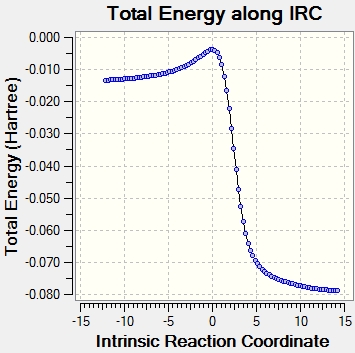 |
| Endo Diels-Alder Reaction |  |
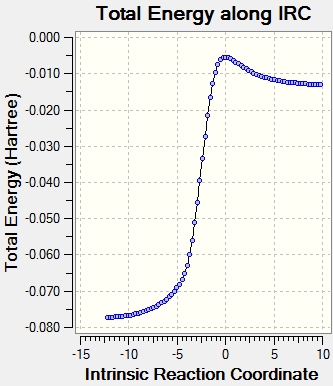 |
| Cheletropic Reaction |  |
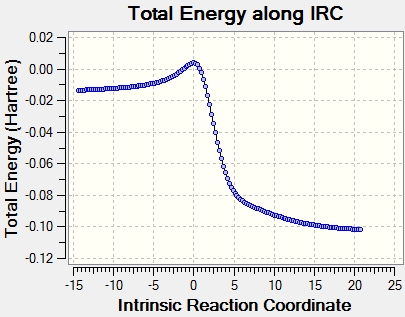 |
A reaction profile containing the energy levels of the reactants, transition states and products from the endo- and exo- Diels-Alder reactions and the cheletropic reaction is shown in figure 25. The cheletropic reaction has the largest activation energy but is the most stabilised which means it is the thermodynamic product. The endo diels-alder reaction has the lowest activation energy which means it is the kinetic product. The exo diels-alder reaction has a higher activation energy and higher gibbs free energy than the endo pathway but is not the preferred route under kinetic or thermodynamic control.
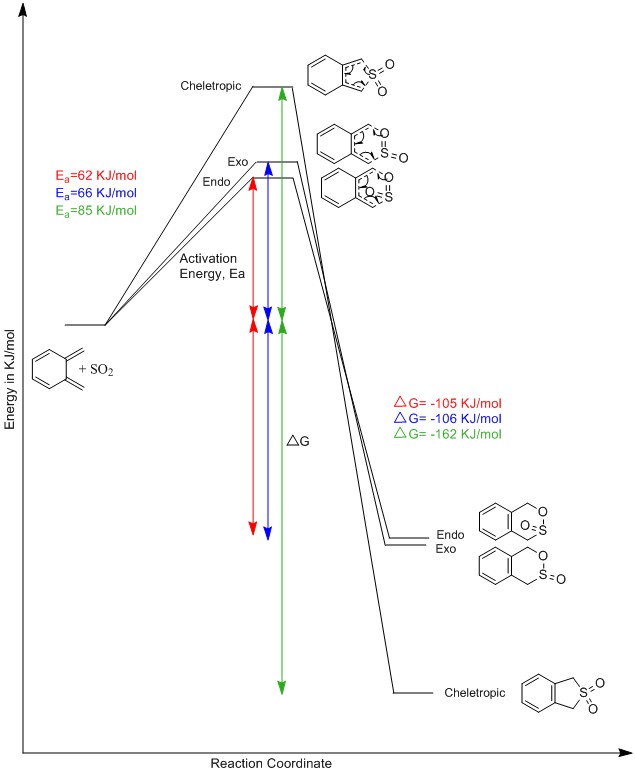
(How did you get these numbers? They don't correspond to any that we have Tam10 (talk) 10:11, 21 February 2017 (UTC))
Conclusion
In these exercises, guassian was used to study the energy and pathways of various reactions. Exercise 1 confirmed that diels-alder reactions are synchronous and a reaction can only occur between orbitals of the same symmetry. Bond formation occurs when the distance between the bonds is smaller than the sum of the van der waals radii of the atoms. Exercise 2 studied the differences between endo- and exo- diels-alder pathways. The endo diels-alder reaction produced the kinetically stabilized product due to the lower activation energy caused by the secondary orbital interactions which do not exist in the exo diels-alder reaction and produced the thermodynamic product due to a greater level of stabilisation due to a more negative gibbs free energy. Exercise 3 showed that a reaction can also proceed via a cheletropic reaction under thermodynamic conditions.
