User:Emw15
Inorganic Computational Labs
(p. Hunt)
AX3
BH3
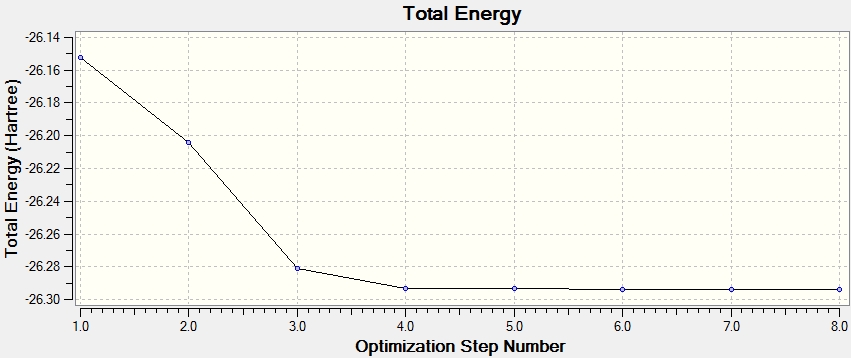
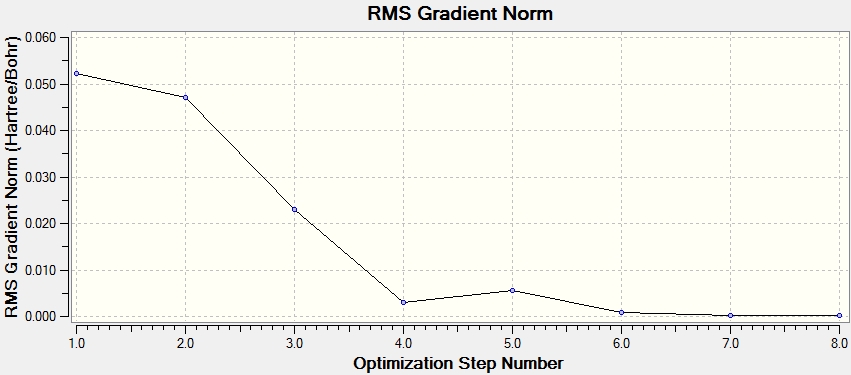
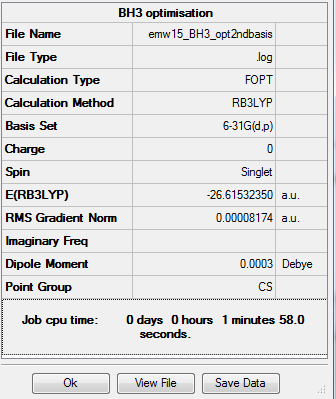
First optimisation
Item table:
Item Value Threshold Converged?
Maximum Force 0.000350 0.000450 YES
RMS Force 0.000178 0.000300 YES
Maximum Displacement 0.000960 0.001800 YES
RMS Displacement 0.000608 0.001200 YES
Predicted change in Energy=-4.408011D-07
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1732 -DE/DX = -0.0002 !
! R2 R(1,3) 1.1723 -DE/DX = 0.0004 !
! R3 R(1,4) 1.1732 -DE/DX = -0.0002 !
! A1 A(2,1,3) 120.0012 -DE/DX = 0.0 !
! A2 A(2,1,4) 120.0016 -DE/DX = 0.0 !
! A3 A(3,1,4) 119.9972 -DE/DX = 0.0 !
! D1 D(2,1,4,3) 180.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
6-31G optimisation
Item Table:
Item Value Threshold Converged?
Maximum Force 0.000203 0.000450 YES
RMS Force 0.000098 0.000300 YES
Maximum Displacement 0.000653 0.001800 YES
RMS Displacement 0.000415 0.001200 YES
Predicted change in Energy=-1.436188D-07
Optimization completed.
-- Stationary point found.
----------------------------
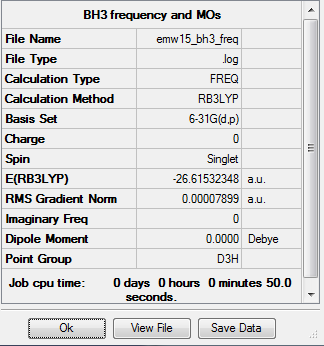
frequency
test molecule |
Full mass-weighted force constant matrix:
Low frequencies --- -0.2456 -0.1129 -0.0054 44.0270 45.1846 45.1853
Low frequencies --- 1163.6049 1213.5924 1213.5951
Diagonal vibrational polarizability:
0.7195224 0.7194221 1.8382230
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A2" E' E'
Frequencies -- 1163.6049 1213.5924 1213.5951
Red. masses -- 1.2531 1.1072 1.1072
Frc consts -- 0.9996 0.9607 0.9607
IR Inten -- 92.4860 14.0835 14.0871
Atom AN X Y Z X Y Z X Y Z
1 5 0.00 0.00 0.16 0.00 0.10 0.00 -0.10 0.00 0.00
2 1 0.00 0.00 -0.57 0.00 0.08 0.00 0.81 0.00 0.00
3 1 0.00 0.00 -0.57 -0.39 -0.59 0.00 0.14 0.39 0.00
4 1 0.00 0.00 -0.57 0.39 -0.59 0.00 0.14 -0.39 0.00
4 5 6
A1' E' E'
Frequencies -- 2580.1517 2713.1166 2713.1178
Red. masses -- 1.0078 1.1273 1.1273
Frc consts -- 3.9530 4.8893 4.8893
IR Inten -- 0.0000 126.4041 126.3946
Atom AN X Y Z X Y Z X Y Z
1 5 0.00 0.00 0.00 0.11 0.00 0.00 0.00 0.11 0.00
2 1 0.00 0.58 0.00 0.02 0.00 0.00 0.00 -0.81 0.00
3 1 0.50 -0.29 0.00 -0.60 0.36 0.00 0.36 -0.19 0.00
4 1 -0.50 -0.29 0.00 -0.60 -0.36 0.00 -0.36 -0.19 0.00
Vibrational Modes:
6 as expected!

Ng611 (talk) 16:33, 29 May 2018 (BST) You should include the symmetries of the modes, as well as their assignment (symmetric stretch etc.). This is data that you couldn't get from the calculation, but would have to generate yourself.
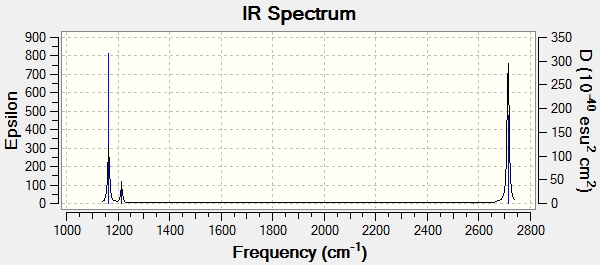
IR Spectrum:
Ng611 (talk) 16:31, 29 May 2018 (BST) There are 6 vibrational modes in the table, but only 3 in the IR spectrum. Why is this?
MOs of BH3
note that the MO diagram used was sourced from figure 5 http://www.huntresearchgroup.org.uk/teaching/teaching_MOs_year2/P1_BH3_MO_diagram.pdf
Ng611 (talk) 16:34, 29 May 2018 (BST) Good analysis. Where is your a1' bonding MO though?
The MOs calculated using the visualised on Gaussian reflected the expected MO drawings accurately. However for the a'1 antibonfinf MO, the lobes drawn on the MO diagram were represented to be smaller than the ones calculated.
Ng611 (talk) 16:35, 29 May 2018 (BST) Which lobes specifically? More importantly, what do these lobes represent? You've done well to spot that there is a discrepancy but you need to expand on this further.
Key information for NH3
Relevant information from item table:
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000012 0.001800 YES
RMS Displacement 0.000008 0.001200 YES
Predicted change in Energy=-9.844518D-11
Optimization completed.
-- Stationary point found.
----------------------------
Frequency analysis:
test molecule |
Low frequencies --- -8.5223 -8.4750 -0.0029 0.0335 0.1918 26.4067 Low frequencies --- 1089.7616 1694.1862 1694.1866
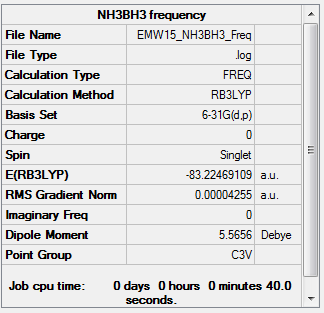
NH3BH3
Key information for NH3BH3
note before moving on to frequency analysis, constrain point group to c3v.
Item Value Threshold Converged?
Maximum Force 0.000189 0.000450 YES
RMS Force 0.000050 0.000300 YES
Maximum Displacement 0.000884 0.001800 YES
RMS Displacement 0.000308 0.001200 YES
Predicted change in Energy=-1.603063D-07
Optimization completed.
-- Stationary point found.
----------------------------
Frequency analysis:
test molecule |
Low frequencies --- -19.0249 -0.0628 -0.0410 0.0482 15.1389 15.3494 Low frequencies --- 262.7369 632.6342 639.0151
Association and dissociation energies
E(NH3)= -26.61532 a.u.
E(BH3)= -56.55777 a.u.
E(NH3BH3)= -83.22469 a.u.
Calculating the association energyː
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] = -83.22469 - (-26.61532 + -56.55777) = -0.05216 a.u. = -135 kJ/mol
Calculating the dissociation energyː
ΔE=[E(NH3)+E(BH3)]-E(NH3BH3)= (-26.61532 + -56.55777)- ( -83.22469)= 0.05216 a.u. = 135 kJ/mol
Ng611 (talk) 16:36, 29 May 2018 (BST) Remember to include some literature bond values (ideally from a textbook, databook, or paper) to compare the strength of the orbital to.
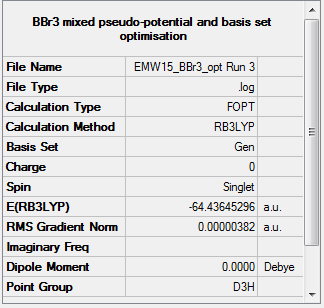
BBr3
Item Value Threshold Converged?
Maximum Force 0.000008 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000036 0.001800 YES
RMS Displacement 0.000023 0.001200 YES
Predicted change in Energy=-4.027594D-10
Optimization completed.
-- Stationary point found.
----------------------------
Frequency analysis for BBr3
test molecule |
Low frequencies --- -0.0137 -0.0064 -0.0047 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
Project: Aromaticity
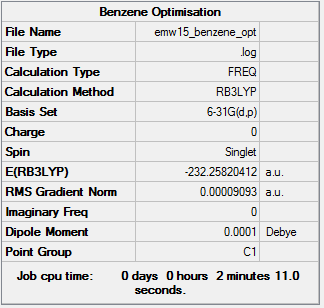
benzene
Item Value Threshold Converged?
Maximum Force 0.000198 0.000450 YES
RMS Force 0.000082 0.000300 YES
Maximum Displacement 0.000849 0.001800 YES
RMS Displacement 0.000305 0.001200 YES
Predicted change in Energy=-4.741875D-07
Optimization completed.
-- Stationary point found.
----------------------------
Benzene Frequency analysis: File:EMW15 BENZENE FREQUENCY.LOG
Low frequencies --- -0.0002 0.0004 0.0008 53.3358 56.8939 57.4988 Low frequencies --- 421.7957 421.8978 626.1055
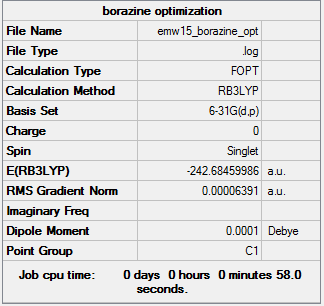
borazine
Item Value Threshold Converged?
Maximum Force 0.000085 0.000450 YES
RMS Force 0.000033 0.000300 YES
Maximum Displacement 0.000243 0.001800 YES
RMS Displacement 0.000077 0.001200 YES
Predicted change in Energy=-9.288705D-08
Optimization completed.
-- Stationary point found.
----------------------------
Borazine frequency analysis:
File:EMW15 BORAZINE FREQ.LOG
Low frequencies --- -13.6263 -7.3156 -0.0004 0.0006 0.0009 11.8128 Low frequencies --- 288.6704 290.1363 404.2329
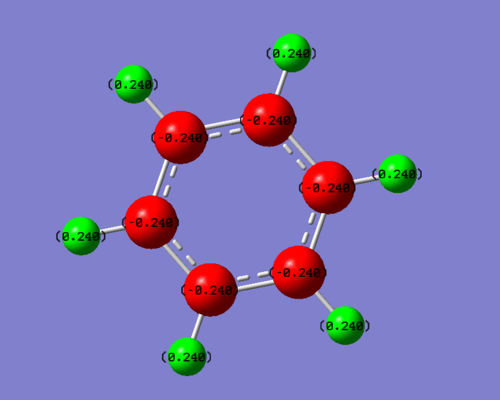
Benzene and Borazine charge analysis
Benzene:
Summary of Natural Population Analysis:
Natural Population
Natural -----------------------------------------------
Atom No Charge Core Valence Rydberg Total
-----------------------------------------------------------------------
C 1 -0.24015 1.99908 4.22747 0.01360 6.24015
C 2 -0.24020 1.99908 4.22752 0.01360 6.24020
C 3 -0.24016 1.99908 4.22749 0.01360 6.24016
C 4 -0.24015 1.99908 4.22748 0.01360 6.24015
C 5 -0.24021 1.99908 4.22753 0.01360 6.24021
C 6 -0.24016 1.99908 4.22748 0.01360 6.24016
H 7 0.24017 0.00000 0.75841 0.00142 0.75983
H 8 0.24016 0.00000 0.75842 0.00142 0.75984
H 9 0.24018 0.00000 0.75840 0.00142 0.75982
H 10 0.24018 0.00000 0.75840 0.00142 0.75982
H 11 0.24017 0.00000 0.75841 0.00142 0.75983
H 12 0.24017 0.00000 0.75841 0.00142 0.75983
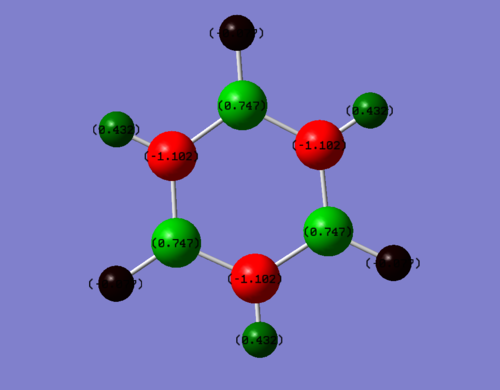
Borazine:
Summary of Natural Population Analysis:
Natural Population
Natural -----------------------------------------------
Atom No Charge Core Valence Rydberg Total
-----------------------------------------------------------------------
H 1 -0.07654 0.00000 1.07585 0.00069 1.07654
H 2 0.43199 0.00000 0.56573 0.00228 0.56801
H 3 -0.07654 0.00000 1.07585 0.00069 1.07654
H 4 0.43199 0.00000 0.56573 0.00228 0.56801
H 5 -0.07654 0.00000 1.07585 0.00069 1.07654
H 6 0.43198 0.00000 0.56574 0.00228 0.56802
B 7 0.74696 1.99917 2.23867 0.01521 4.25304
B 8 0.74698 1.99917 2.23865 0.01520 4.25302
B 9 0.74698 1.99917 2.23865 0.01520 4.25302
N 10 -1.10242 1.99943 6.09821 0.00478 8.10242
N 11 -1.10240 1.99943 6.09820 0.00478 8.10240
N 12 -1.10241 1.99943 6.09821 0.00478 8.10241
=======================================================================
Ng611 (talk) 16:40, 29 May 2018 (BST) Remember to use identical colour scales for both molecules.
Looking at the diagrams, Benzene and borazine are both aromatic as they both obey Huckel’s 4n+2 rule on aromaticity. As shown on the charge distribution visualizer on Gaussian, the more electronegative atoms are coloured in red while the more electropositive ones are green. The intermediate black atoms in borazine correspondingly represents an intermediate charge close to zero, meaning almost neutral.
Due to its aromaticity and symmetrical shape, all the carbons and hydrogens in benzene are equivalent. All the 6 carbons and all 6 of the hydrogens have the same charge, at -0.240 and +0.240 respectively. Since carbon is more electronegative than hydrogen in the contiguous delocalised pi system it takes the negative charge.
Borazine is less symmetric than benzene and is comprised of more electronegative nitrogen atoms replacing 3 of the carbons in benzene and 3 boron atoms replacing the other 3 carbons in an alternating fashion. The added electronegativity difference gives the bonds in borazine a more ionic character than those in benzene. Thus, the delocalisation of the pi system in borazine is less complete than in benzene.
Taking these factors into account, although benzene and borazine are isostructural, the benzene molecule is non-polar while borazine is polar.
Ng611 (talk) 16:40, 29 May 2018 (BST) What do you mean when you say polar (presumably, you mean that there's an overall dipole, but try and be as specific as possibe). What about the overall charge of the molecule? What about the summation of charges for the B-H/N-H bonds?
bonding analysis
Ng611 (talk) 16:42, 29 May 2018 (BST) Well done for comparing the correct MOs by shape and not energtic ordering (which is not necessarily reliable). I would include a brief discussion of the overall symmetry and electronegativity differences between the atoms in the molecule to improve this section further. Perhaps also consider dicussing the constituent AOs that form the MOs and the overall symmetry of the MO.
aromaticity discussion
Per Huckel’s rules which are used to define an aromatic molecule, the molecule must have 4n+2 pi electrons in a contiguous array in adjacent p orbitals in a planar arrangement to allow the pi electrons to circulate effectively. This form of resonance enables the benzene and borazine molecules to achieve enhanced stabilisation. To prove that a molecule is aromatic, experimental techniques such as calorimetry can be used to observe intermediate bond lengths between the carbons in benzene or boron and nitrogen in borazine. Since the electrons are shared in a conjugated pi system, there shouldn’t be alternating double and single bonds, instead there will be intermediate bond lengths. Additionally, using 1HNMR spectroscopy for aromatic compounds there are two different proton environments for protons lying inside and outside the ring due to being shielded and de-shielded because of the circulating pi cloud in the aromatic molecules.
The molecular orbital theory for benzene can be explained using the Linear Combination of Atomic Orbitals (LCAO). The s-bonding framework for the contiguous carbon ring is comprised of sp2 hybridized carbon atoms, leaving the pz orbital (orthogonal to the plane of the ring) in the appropriate orientation to allow pi electrons to circulate in this contiguous ring above and below the plane. The 6 electrons present in the aromatic ring are arranged per the Aufbau Principle. The lowest energy MOs are filled first putting the electrons in the bonding orbitals, leaving thr antibonding MOs empty. Thus all the pi orbitals in benzene are in phase in the lowest energy MO.
Borazine is isostructural to benzene and unsurprisingly has similar properties. For instance, equivalent N-B bond lengths and 6 pi electrons, following the 4n+2 rule can also be observed for borazine. While borazine is still so aromatic, it is less energetically stabilised and exhibits poorer aromaticity than benzene due to the more electronegative B and N atoms which create a dipole and enrich the ionic character of the bonding in borazine. This makes the electron delocalisation less complete and so less aromatic.
Ng611 (talk) 16:46, 29 May 2018 (BST) A good basic overview of aromaticity here. Recent studies have caused us to begin to rethink our view of aromaticity somewhat however. Some thoughts on how the modern coneptual model of aromaticity differs from Huckel's model would improve the section further.
Ng611 (talk) 16:46, 29 May 2018 (BST) Some very good calculations in this report. Some additional attention should be paid to the layout of your wiki, and remember to include all of the job information in a separate subheading. Also, consider adding some additional discussion to your benzene/borazine MO analysis and your final discussion on aromaticity. You have good result -- try and show them off as much as you can.