User:Ds2909
Module 1
The Hydrogenation of Cyclopentadiene Dimer

Cyclopentadiene dimerises specifically to produce endo dimer 2, instead of exo dimer 1. This is known as the endo rule. The hydrogenation of this dimer produces initially only one of the dihydro derivatives 3 and 4. In this project these 4 molecules are examined using MM2 and their reactivity rationalised.
| Type/kcal | Exo dimer 1 | Endo dimer 2 |
| Stretch: | 1.28 | 1.25 |
| Bend: | 20.58 | 20.84 |
| Stretch-Bend: | -0.84 | -0.83 |
| Torsion: | 7.65 | 9.51 |
| Non-1,4 VDW: | -1.42 | -1.54 |
| 1,4 VDW: | 4.23 | 4.31 |
| Dipole/Dipole: | 0.37 | 0.44 |
| Total Energy: | 31.87 kcal | 33.99 kcal |
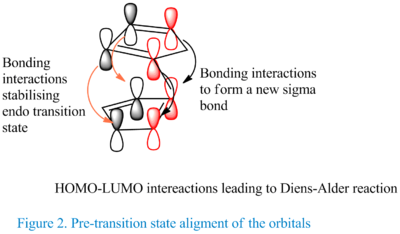
By looking at the relative energies, the exo dimer is lower in energy by 2.12 Kcal/mol mostly due to a lower torsional strain (i.e. deviations from optimal dihedral angles). This is indicative that the observed endo product is a kinetic product. Its formation can be explained in terms of secondary orbital overlap stabilising the endo transition state, Figure 1.
| Type | Hydrogenation isomer 3 | Hydrogenation isomer 4 |
| Stretch: | 1.24 | 1.09 |
| Bend: | 19.15 | 14.52 |
| Stretch-Bend: | -0.83 | -0.54 |
| Torsion: | 11.07 | 12.49 |
| Non-1,4 VDW: | -1.64 | -1.06 |
| 1,4 VDW: | 5.79 | 4.51 |
| Dipole/Dipole: | 0.16 | 0.14 |
| Total Energy: | 34.96 kcal/mol | 31.15 kcal/mol |
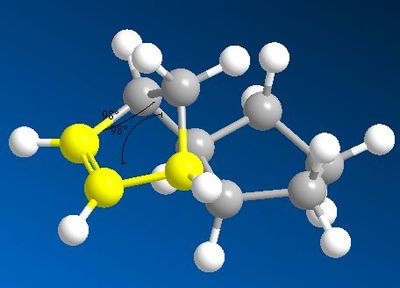
By looking at the relative energies, dihydro product 4 is lower in energy than 3 by 3.8 kcal/mol. The main difference arises from the Bend contribution (deviations from ideal bond angles). The main factor contributing to the strain in compound 3 is the fact that the alkene is in a fused ring, which causes the deviations of the olefin bonds from the ideal 120o to 98o cf. 112 and 113 in isomer 4.
Thus from a thermodinamic point of view it is expected that isomer 4 should be hydrogenation initial product.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
An intermediate in the total synthesis of Taxol, compound 9, shows isomerisation to an atropoisomer with the carbonyl group pointing down, compound 10[ref]. Also the alkene moiety in both isomers shows abnormal slow reactivity. The current analysis aims to use MM2 molecular mechanics to rationalise the experimentally found behaviour.
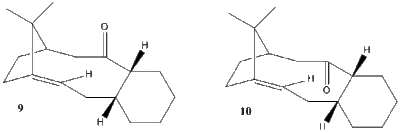
Atropoisomers are molecules in which chirality arises not due to the presence of a stereogenic center but due to restricted rotation around a C-C single bond. In this case the presence of the fused rings, bridgedhead double bond, and carbonyl rigidifies the 9 membered ring so that a barrier to interconversion exists.
An initial optimisation of the structure of the two compounds was performed; the 6 membered rings were set to a chair conformation and the strain around the double bond was minimised by modifying the 9 membered ring.
| Taxol UP | TAXOL DOWN |
|---|---|
 |
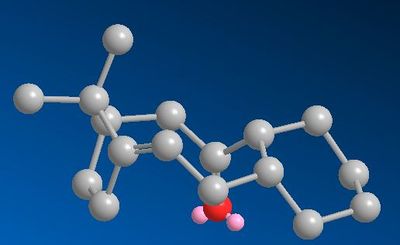 |
| 9-Taxol down | 10-Taxol up | |
| Stretch: | 2.78 | 2.61 |
| Bend: | 16.54 | 11.33 |
| Stretch-Bend: | 0.43 | 0.34 |
| Torsion: | 18.24 | 19.66 |
| Non-1,4 VDW: | -1.55 | -2.15 |
| 1,4 VDW: | 13.11 | 12.87 |
| Dipole/Dipole: | -1.72 | -2.00 |
| Total Energy: | 47.83 kcal/mol | 42.68 kcal/mol |
It can be seen that the ‘down’ isomer, 9, is more stable by about 5 Kcal/mol. The biggest contribution to the difference in energy is the Bending strain, i.e. deviation from ideal bond angles. By looking at the two 9 membered rings, the Endo isomer is in a chair-boat conformation, whereas the exo isomer is in a more energetically expensive boat-twist boat conformation. Thus from a thermodynamical point of view the exo isomer should convert to the endo isomer to relieve bending strain in the 9 membered ring. Further optimisation of the 9 membered ring was not possible due to it being rigidified by the presence of two fused rings, a bridged double bond and a sp2 carbonyl carbon.
Modifying the cyclohexane ring into a twist boat conformation was energetically unfavourable for both structures; although for the exo isomer the energetic penalty for to lock the 6 memebered ring into a twist boat conformation to relieve the strain is not that …
Finally the MM2 optimised geometries were submitted for a Geometry optimisation on the mpw1pw91/6-31(d,p) level. This confirmed that the obtained structure for the endo isomer was in fact the global minimum. Interestingly for the exo isomer the result was that the cyclohexane ring was forced into a twist boat conformation to relieve the strain in the 9 membered ring.
The reason why the less stable exo geometry is obtained in first place is due to the electronic requirements of the oxyanionic Cope Rearangement(i.e. a 3.3. sigmatropic rearrangement)which led to compound 9. The TS requires an endo-chiar conformation, figure , leading to the exo atropoisimer.
Wihelm et al defined ‘hyperstable olefins are molecules which contain less strain than their parent hydrocarbon and negative Olefinic Strain Values. Such olefins should be very unreactive – not due to steric hindrance or enhaced π-bond strength but due to special stability afforded by the cage structure and to the greater strain of the parent polycycloalkane.’
By comparing the relative strain (the Bending and especially Torsion) of the alkene and corresponding alkane, the same conclusion can be reached for the two atropoisomers at hand. The bend increases by 2.9 kcal/mol by going from isomer 10 to its corresponding alkane; the torsion by 2.5 kcal/mol and 1,4 VDW by 3.1 kcal/mol. Similarly for molecule 9, bent increases by 1.2 kcal/mol, torsion by 4.9, 1,4 VDW by 3 kcal/mol.
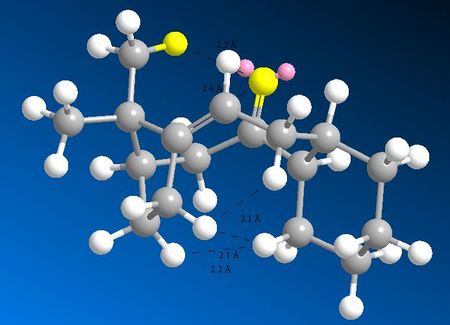
In addition to the torsional strain, there are unfavourable (2.1 A <) VDW interactions. On going from alkene to polycycloalkane, everything gets pushed in the cage, leading to quite a few unfavourable H-H close contact interactions.
| Contribution(kcal/mol) | Molecule 10 | Molecule 10 hydro | Molecule 9 | Molecule 9 hydro |
| Stretch: | 2.61 | 2.84 | 2.78 | 2.97 |
| Bend: | 11.33 | 14.23 | 16.54 | 17.67 |
| Stretch-Bend: | 0.34 | 0.65 | 0.43 | 0.68 |
| Torsion: | 19.66 | 22.11 | 18.24 | 23.1 |
| Non-1,4 VDW: | -2.15 | -2.65 | -1.55 | -1.43 |
| 1,4 VDW: | 12.87 | 15.88 | 13.11 | 16.12 |
| Dipole/Dipole: | -2.00 | -1.73 | -1.72 | -1.77 |
| Total Energy: | 42.68 | 51.36 | 47.83 | 57.30 |
Modelling Using Semi-empirical Molecular Orbital Theory
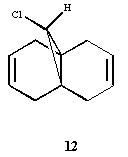
Molecule 12 undergoes regioselective electrophyllic adition to the alkene endo to the chlorine. MOPAC/PM6 is used to calculate the frontier molecular orbitals and rationalise structure and reactivity. The IR spectra is calculated and rationalised using the information found from analysing the Frontier Molecular Orbitals.
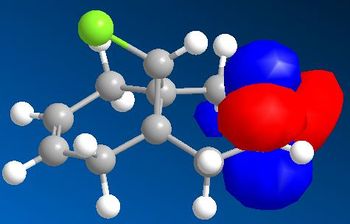
As opposed to the MM2 method which treats the molecule from a ‘classical’ two atoms on a string point of view, the MOPAC/PM6 method takes into account the electronics of the molecule from a quantum mechanical point of view. The obtained frontier molecular orbitals are graphically represented in Figure . The HOMO of the molecule is clearly based on the endo alkene (with very little electron density on the exo alkene), thus explaining regioselectivity when reacting with an nucleophillic reagent such as dichlorocarbene. This is indeed found experimentally [ref].
| HOMO-1 | HOMO | LUMO | ||
|---|---|---|---|---|
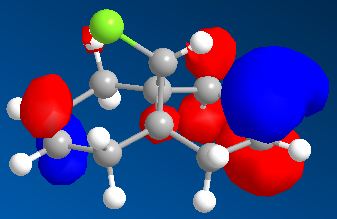 |
 |
 |
||
| LUMO+1 | LUMO+2 |
|---|---|
 |
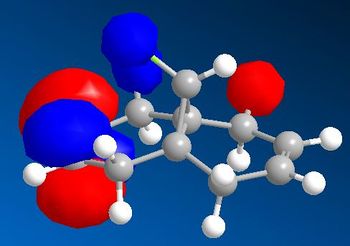 |
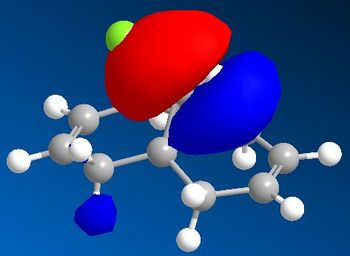
From analysing the other frontier molecular orbitals, overlap between the Cl-C* (LUMO+1) and the alkene π orbital (HOMO-1) can be identified. This would explain why the exo double bond is electron poor compared to the endo double bond. One way to look at this is to consider that even though both interactions lead to weakenging of each bond they also lead to an overall stabilisation of the molecule. From the electronic point of view, a filled orbital interacts with an empty orbital, leading to an overall stabilisation of the system. Thus the exo π orbital is brought down in energy (to HOMO-1) relative to the endo (HOMO). The orbital most likely to react is the HOMO because it is higher in energy, more polarisable and can more readily attack an electrophile.
THe Cl-C o* - exo π interaction should weaken both the C=C and the C-Cl bonds since electrons are taken from a bonding orbital and put into an anti-bonding one. Using the optimised geometry of 12 and its exo dihydro derivative a B3LYP/6-31G(d,p) Gaussian geometry optimization and frequency calculation was performed.
| Molecule 12 | Dihydro derivative |
|---|---|
 |
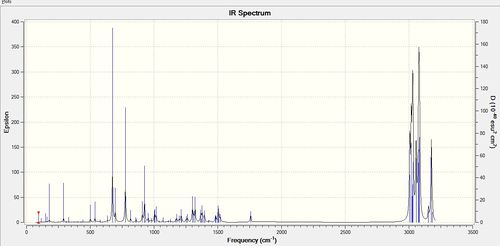 |
| ' | Freq/ cm-1 | Intensity/ ε |
| C-Cl diiene | 770.86 | 25.13 |
| C-Cl dihydro | 775.06 | 19.98 |
| C=C endo diiene | 1757.37 | 3.93 |
| C=C dihydro | 1758.07 | 4.34 |
| C=C exo diiene | 1737.15 | 4.20 |
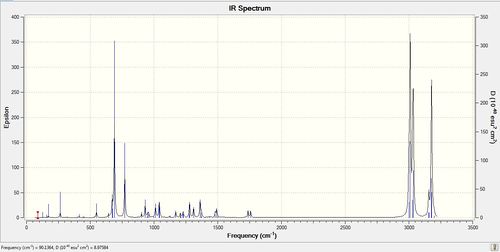
The results correlate well with what is expected. The frequency of the C-Cl stretch increases by 5 cm-1 upon hydrogenation of the exo double bond, corresponding to a slightly stronger bond due to the absence of the electron donation into the C-Cl σ* orbital. The intensity of IR absorptions is proportional to the change in dipole moment of the molecule. Upon hydrogenation, the C-Cl stretch intensity decreases from 25.13 to 19.98ε, corresponding to a more localised change in dipole moment; in the parent compound the change in dipole would also be induced in the exo double bond leading to a better separation of charge(i.e. bigger dipole, larger intensity). The alkene stretches of the two double bonds in compound 12 correlate well with the ideas presented so far. The endo alkene has a higher IR stretching frequency, because it is not involved in donating electron density to chlorine,whereas the exo alkene has a lower stretching frequency again due to donation of its π electrons into the C-Cl σ* antibonding orbital. The intensity of the two stretches again correlate with the idea that the overlap between the endo π - C-Cl σ* leads to a bigger, more spread transition dipole, hence higher intensity (4.20 vs.3.93). Hydrogenation has a minimal effect on the endo alkene as it does not have the correct symmetry to be involved in secondary orbital interactions with the chlorine orbitals.
Monosaccharide chemistry: glycosidation
NGP is one of the most powerful tools in sugar chemistry, as it allows perform glycosidasions diastereospecific. Using MM2 and MOPAC/PM6 the diasteroespcificty is modelled.

First an optimisation is performed on A and B. The chosen R group is the methyl group due to the fact that it will make computational calculations easier without lowering the quality of the result. In synthesis the most accessible form would be the hexa-acylated structure. Modelling this would complicate calculations as it has much more electrons (17 vs 7), dipol-dipol interactions. Similarly a simple H was not chosen due to the H-bonding interactions it would generate and the fact that it is rarely the structure used practically. MOPAC/PM6 is better suited for the calculations as it considers the molecule from a quantum mechanical point of view, thus considering secondary orbital interactions as opposed to MM2 which optimises the molecule from a ‘classical’ point of view of trying the get the least strain and . ….. Still MM2 is a fine method for pre-optimising the geometry for MM6.
With respect to the conformation of the neighbouring acyl group, two conformations A, A`, B, B` can be obtained, with the acyl group pointing above or below the plane of the oxonium cation.
The results of the calculations are presented in Table 1.
| Contribution/kcal/mol | A (Clikc for Jmol) | A`(Click for Jmol) | B | B`(Click for Jmol) |
| Stretch: | 2.45 | 2.35 | 2.55 | 2.45 |
| Bend: | 10.62 | 14.53 | 11.28 | 17.22 |
| Stretch-Bend: | 0.82 | 0.95 | : 1.00 | 1.16 |
| Torsion: | 0.56 | 0.27 | T1.91 | 1.86 |
| Non-1,4 VDW: | 0.07 | -1.70 | : -1.43 | -2.25 |
| 1,4 VDW: | 18.87 | 18.55 | 18.31 | 17.51: |
| Charge/Dipole: | -4.13 | -8.12 | : -7.5 | 4.05 |
| Dipole/Dipole: | 7.80 | 7.37 | : 4.9314 | 4.90 |
| Total Energy: | 17.09 | 34.21 | 31.01 | 46.92 |
| MM6 heat of form | -94.2 | -79.7 | -88.73 | -77.76 |
The main comparison data point should be the MM6/MOPAC heat of formation, as it takes into account secondary orbitals overlaps. It can be seen that the conformations in which the acyl oxygen point towards the oxonium ion, A and B are more energetically favourable (by 14.5 kcal for A-A`, and 11 for B-B`) than it pointing away in A` and B`. Even though there is no formal bond between the acyl oxygen and the oxonium ion, MOPAC, in A and B, actually forms an dioxelenium ion; the oxygen is placed 1.6 A (i.e. bonding distance) from the oxonium ion. This is a stabilising interaction and accounts for the 12.1 and 14. Kcals per mole.
| Contribution/kcal/mol | C(click for Jmol) | C`(click for Jmol) | D(click for Jmol) | D`(click for Jmol) |
| Stretch: | 2.1 | 2.7 | 1.9 | 2.6 |
| Bend: | 14.3 | 18.1 | 18.9 | 17.2 |
| Stretch-Bend: | 0.8 | 0.8 | 0.8 | 0.8 |
| Torsion: | 7.5 | 8.9 | 8.4 | 9.1 |
| Non-1,4 VDW: | -3.4 | -2.8 | -2.1 | -2.7 |
| 1,4 VDW: | 17.7 | 19.4 | 17.4 | 19.9 |
| Charge/Dipole: | 1.5 | 5.5 | -9.4 | -3.0 |
| Dipole/Dipole: | -0.6 | 0.2 | -0.2 | -1.1 |
| Total Energy: | 39.8 | 52.6 | 35.6 | 42.8 |
| MM6 heat of form | -94.5 | -60.1 | -88.6 | -66.9 |
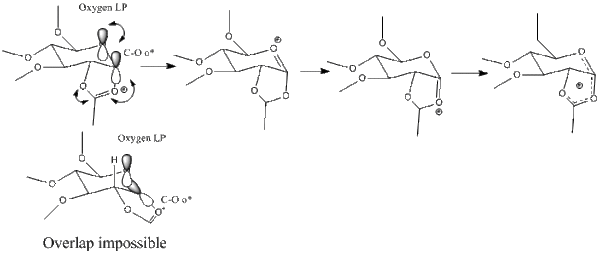
The results above correlate well with what is expected. Molecule C has the same energy as molecule A, and D the same as B. This is because the MOPAC/PM6 does not distinguish between formal bonds and secondary interactions; it minimises the energy based on the lowest energy electronics, and it arranged the atoms in A, just as in C, (and the ones in B just as in D), effectively putting a bond between the acyl group and the oxonium ion and showing that the dioxolenium form is actually the one found experimentally not the oxonium one.
C` is much less stable than C (by by 34.4 kcal/mol), and D` is much less stable than D (by 21 kcal/mol). This is due to the anomeric effect, which can only occur when the substituents are axial (i.e. C and D but not C` and D`). Even though both C` and D` could be attacked from either above or below from an incoming nucleophile, because of the very large barrier to interconversion, they are virtually non existent. Boltzman distribution can be used to calculate the ration between C/C` and D/D`= exp(-(-ΔE/kT)) = 10^60 (i.e. virtually only D exist). The number is event larger for the C/C ratio, .. For the case of C and D, an incoming nucleophile could only attack at the trans position relative to the acyl group, as the axil acyl group/dioxolonium ion in C and D,protects the other side. Thus this would explain the diastereospecificity of the glycosadatiion when NGP is used.
Mini Project -Stereoselective dissolving metal reductions
In a recent natural product synthesis, molecule 5 was reduced stereoselectively to 6 using a Birch Reduction (Li in liq. NH3). This project aim is to confirm that the reported isomer is the correct one by predicting the 13C and IR spectra, Optical Rotatino, and 3H coupling constanst.
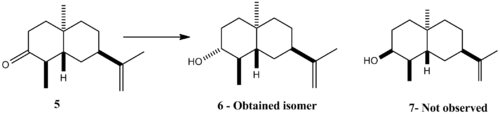
In order to determine if the reaction has worked, i.e. if the ketone was reduced, the spectroscopic data should reveal the necessary information. The ketone 13C NMR signal is expected to appear at about 200ppm, whereas the C-OH peak at about 70ppm. Looking at the published NMR data it is clear that there is no ketone signal but a C-OH signal at 76.8ppm. IR should also provide satisfactory data: there should be no carbonyl peak at 1750 region and there isn’t, insteat there is the O-H stretch at 3400. In the NMR, an extra proton should appear together with its corresponding coupling constants to the neighbouring protons.It can be found in the paper at 3.10 ppm.
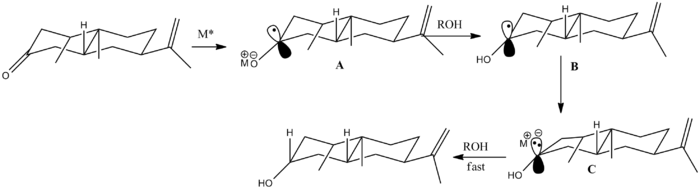
Mechanism leading to the stereoselective Birch reduction in shown on the right. The first step is a single electron transfer to form radical anion A. This radical anion is planar and it is resonance stabilised with about 70% of the electron density at carbon [ref]. The next step is protonation (from the presence of the alcohol as proton donor) to give a hydroxy carbon radical B in which piramidalisation has occured to a considerable extent. The hydroxyl group is now oriented almost exclusively in an equatorial position[ref]. Another single electron transfer takes place to form the anion C, which undergoes a fast protonation to give equatorial alcohol in >99% yield. Thus this explains the steoreslectivity of the reaction.
Predicting and comparing the 13C spectra
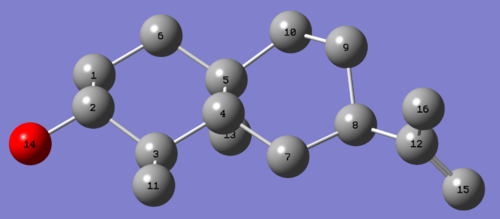
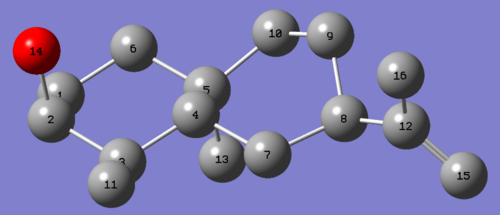
Simulating the 13C spectra:After a pre-optimisation using MM2 and MOPAC/MP6, the molecule was sent to SCAN for geometry optimisation at the mpw1pw91/6-31g(d,p) level. The 13C spectra was predicted using the GIAO approach. Results are displayed in table 1.
| Molecule 6 | Molecule 7 |
|---|---|
 |
 |
| Carbon | Calculated Mol 6 | Lit. values 6 | Relative Error | Calculated Mol 7 | Literature | Relative error |
| 12 | 147.3 | 147.1 | -0.2 | 147.5 | 147 | -0.5 |
| 15 | 109.3 | 110.7 | 1.4 | 109 | 110.8 | 1.8 |
| 2 | 74.25 | 76.8 | 2.55 | 71.6 | 72.2 | 0.6 |
| 3 | 41.2 | 38.88 | -2.32 | 41.95 | 39.17 | -2.78 |
| 8 | 41.7 | 39.9 | -1.8 | 39.09 | 37.62 | -1.47 |
| 6 | 41.4 | 39.3 | -2.1 | 37.83 | 37.23 | -0.6 |
| 4 | 42.3 | 43.22 | 0.92 | 37.28 | 35.5 | -1.78 |
| 10 | 39.1 | 37.1 | -2 | 33.25 | 35.27 | 2.02 |
| 5 | 33.5 | 33.78 | 0.28 | 33.6 | 34.04 | 0.44 |
| 7 | 30.9 | 26.1 | -4.8 | 29.9 | 29.01 | -0.89 |
| 1 | 30.6 | 30.9 | 0.3 | 26.4 | 25.9 | -0.5 |
| 9 | 26 | 23.1 | -2.9 | 26.1 | 23.28 | -2.82 |
| 13 | 18.7 | 16.68 | -2.02 | 25.6 | 22.81 | -2.79 |
| 16 | 24.1 | 22.82 | -1.28 | 18.7 | 15.85 | -2.85 |
| 11 | 15.4 | 14.87 | -0.53 | 16.3 | 15.76 | -0.54 |
AVG ERROR 6 = 1.69 AVG ERROR 7 = 1.49
By looking at the two structures the two rings are in the same chair and twist boat conformation with the main difference being the orientation of the OH group. Thus the main signals expected to be different are the ones from C2 (which bears the hydroxyl group), the adjacent C1 and C3, and the rest of the carbons in the same ring C4, C5, C6. Assignment of signals was taken from the paper, where 2D NMR and NOE was used to unambiguously assign each signal.
The calculated 13C data correlates well with the literature data (average error is 1.69ppm and maximum error 0f 2.55ppm for 6 and 1.49ppm and 2.85 for 7) and can be used to confirm unambiguously that the obtained structure is the correct one. For the case of the C2 carbon bearing the hydroxyl group the predicted value for isomer 6, 74.25 ppm correlates with the literature value of 76.8 ppm, albeit with a relative large error; the predicted C" value for isomer 7, 71.6pm correlates well with the 72.2 ppm value from literature. Similarly the predicted values for the C1 correlate well with experimental data( for molecule 6: 30.6 predicted -30.9 lit vs.26.4 -25.9 for molecule 7), further confirming that the reported isomer is indeed the one obtained. Table 2 provides a list of selected result which unambiguously determine that the obtained configuration matches the reported one.
| Carbon | Calc 13C Obt iso | Lit. obt iso | Error | Calc 13C other iso | Lit other iso | Error |
| 2 | 74.25 | 76.8 | 2.55 | 71.6 | 72.2 | 0.6 |
| 6 | 41.4 | 39.3 | -2.1 | 37.83 | 37.23 | -0.6 |
| 4 | 41.2 | 43.22 | 2.02 | 37.28 | 35.5 | -1.78 |
| 1 | 30.6 | 30.9 | 0.3 | 26.4 | 25.90 | -0.5 |
Normal 0 false false false EN-GB X-NONE X-NONE
Interestingly some changes in the 13C shifts happen in the other ring too, even though the geometry and connectivity seems unchanged. These probably arise from the resulting change in Molecular Orbitals which are probably not taken into account by the used method and basis set.
| Carbon | Calc 13C Obt iso | Lit 1 obt iso | Error | Calc 13 C other iso | Lit other iso | Error2 |
| 13 | 18.7 | 16.68 | -7.42 | 25.6 | 22.81 | -2.79 |
| 16 | 24.1 | 22.82 | 4.12 | 18.7 | 15.85 | -2.85 |
| 7 | 30.9 | 26.1 | -4.8 | 29.9 | 29.01 | -0.89 |
| 8 | 41.7 | 39.9 | -1.8 | 39.09 | 37.62 | -1.47 |
Analysing the 3J coupling constants
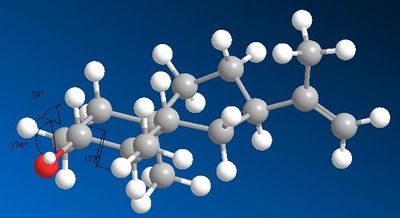
More compelling evidence that the obtained isomer is the assigned one is provided by the 3J coupling constants. Even though coupling constants are reported for only 2 protons (the others being unresolved multiples) one is particularly useful to make an assignment. The coupling constants for H2 are reported. For isomer 6, H2 couples with 3 other protons, 2 of which are almost anti-periplanar and one at 60oC . Using Jannochio to predict the coupling constants:
| Molecule 6 | Molecule 7 |
|---|---|
 |
 |
| Atoms | Angle | Predicted J | Experimental J | ' |
| H2-H1 | -59.0 | 4.26 | 5.1 | |
| H2-H1` | -174.2 | 11.22 | 11.0 | |
| H2-H3 | 178.1 | 9.78 | 9.5 | |
These coupling constant are in good agreement with the ones obtained in the paper, confirming that the assignment is indeed correct in the paper. The corresponding J values for the other isomer are very different due to the equatorial H2, and the predicted coupling constant fall between 2.12 and 3.25 (unresolved multiple in paper).
| Atoms | Angle | Predicted J | Exp J |
| H2-H1 | -51.5 | 2.96 | N/A |
| H2-H1` | 64.5 | 3.25 | N/A |
| H2-H3 | 55.0 | 2.12 | N/A |
|
|
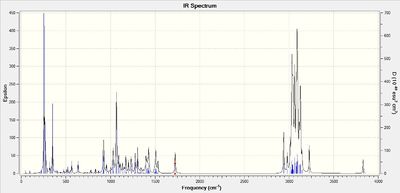
IR spectra
The reported IR spectra is very poor, with only 5 unassigned peaks. Nevertheless the computed spectra is included below, together with the reported literature data.
| IR of molecule 6 | IR of molecule 7 |
|---|---|
 |
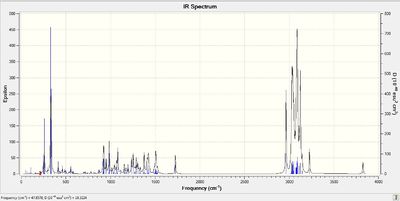 |
Lit IR of 6: IR (CHCl3) ν Normal 0 false false false EN-GB X-NONE X-NONE max 3620, 3472, 3096, 1640, 896 cm-1 Lit IR of 7: IR (CHCl3) νmax 3416, 1642, 890 cm-1
Optical Rotation
The literature value for the optical rotation is -11.6 for compound 6 and +19.7 for compound 7. Only compound with an optical rotation >|100| can be predicted with confidence. The computed values are -106.8 for 6 and 86.9 for 7, values which are quite off but which are correct in absolute sign, further aiding in assigning the structure.
Discarded mini-project:Regio- and stereoselective conversion of alkenes to epoxides
Initially this project was chosen and calculations were attempted. Due to the lack of spectroscopic data, one of the post graduate demonstrators, Bao Nguyen advised that the project be abandoned and a new mini-project and attempt another. Bao also suggested that the work that has been done be posted for the extra 5% credit given to students for finding their own project.
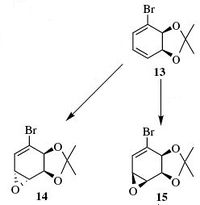
In a recent paper concerned with making intermediates useful in natural product synthesis, compound 13 was shown to regioselectively and stereoselectively epoxidise to give either 14 or 15. The aim of this project is to use computational methods to explain the regioselectivity and to confirm that the assignment in the paper is correct.
Analysing the regioselectivity An analysis similar to the one in question 3 was attempted. MM2 was used to pre-optimised structure 13, after which a MOPAC/PM6 calculation was attempted. The HOMO was then visualised. By looking at the relative size of the orbitals on the two alkenes, it can be seen that the opposite results to what was expected was obtained; the more electron rich alkene seems to be the one with the Br atom.
| HOMO of molecule 13 | Stability of carbocations |
|---|---|
 |
 |
Similar studies have been conducted in literature and the explanation given by [ref] is that the corresponding cation 9 resulting by attack at the C=C-Br alkene would not enjoy the same resonance stabilisation as cation 10. Since both transformation of 13 to 14 and 13 to 15 are electrophilic reactions the same arguments apply.
Analysing the stereoselectivity
The reagent used for the transformation of 13 to 14 is m-CPBA in CH2Cl2. The reaction is a concerted one, and theoretically the alkene can be attacked from either above or below. In this case here the approach from the α-face is blocked by one of the methyl groups of the pinacol protecting group.
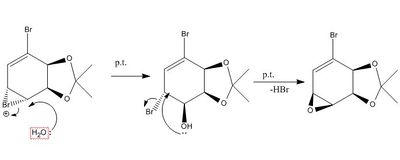
The reagent used in the transformation of 13 to 15 is NBS in THF-H2O, which is again an electrohpillic reaction, proceeding via a bromonium ion. Initially the bromonium ion should form on the endo face (due to the same steric arguments as the epoxidation reaction). The bromonium ion is then attacjed by water from the top side to form a halo alcohol, which is then set up to eliminate HBr to arrive at the endo epoxide 15.
Predicting the 13C spectra
| Molecule 14 opt geo | Molecule 15 opt geo |
|---|---|
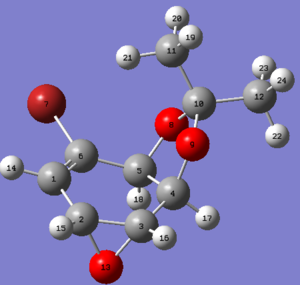 |
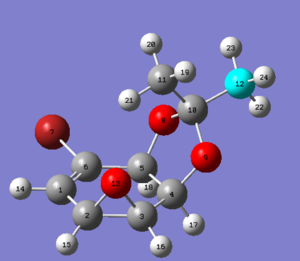 |
| Carbon | CMPD 14 calculated/ppm | CMPD 14 lit values/ppm | CMPD 14 Error | CMPD 15 calculted/ppm | CMPD 15 lit values | CMPD 15 error |
| 6 | 138.1 | 137.9 | -0.2 | 131.6 | 129.9 | -1.7 |
| 1 | 123.1 | 122.2 | -0.9 | 127.1 | 126.5 | -0.6 |
| 10 | 110.3 | 111.4 | 1.1 | 110.3 | 111.4 | 1.1 |
| 5 | 73.4 | 73.1 | -0.3 | 74.7 | 74.2 | -0.5 |
| 4 | 72.2 | 72.6 | 0.4 | 72.3 | 72.7 | 0.4 |
| 3 | 49.5 | 49.6 | 0.1 | 49.5 | 49.5 | 0 |
| 2 | 48.3 | 47.9 | -0.4 | 48.5 | 48.3 | -0.2 |
| 11 | 27.4 | 27.4 | 0 | 27.9 | 27.5 | -0.4 |
| 12 | 26.1 | 25.8 | -0.3 | 26.4 | 26.0 | -0.4 |
It can be seen that the predicted data is in good agreement with the data in literature. The average error for compound 14 calculations is 0.6ppm, the maximum error being 1.1 ppm. For compound 15 ithe average error is only 0.41 ppm with the maximum error 1.1ppm. The C6 is pretty much the only shift that can distinguish between the isomers, all the other being very close. It can be seen that the predicted values for C6 indicate that the assignment in the literature is correct (for molecule 14 the predicted C6 shift is 138.1ppm vs 137.9 lit value; for molecule 15 the predicted shift is 131.6ppm vs 129.9 lit value).
Analysing the 3J coupling constants
Due to the fact that the cyclohexane ring is almost flat in both molecule due to the presence of two fused rings and a double bond; there is very little difference between the 3J coupling of the 2 compounds. Also the NMR signals are not assigned in any of the literature paper, making it impossible to use the 3J coupling constants supplied (which are very similar and thus unusable in the first hand).
| Dihedral angles 14 | Dihedral angles 15 |
|---|---|
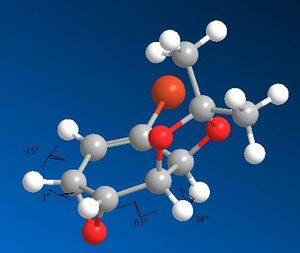 |
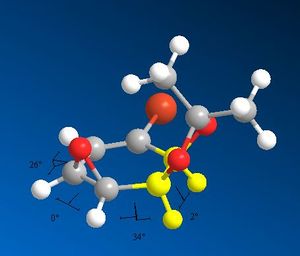 |
