User:Ck3112
The Cope Rearrangement Tutorial
The cope rearrangement is a pericyclic reaction, it is a [3,3] sigmatropic rearrangement of 1,5-dienes. In this experiment the cope rearrangement of 1,5-hexadiene is examined.
Optimizing the Reactants and Products
Firstly 1,5-hexadiene was drawn in the antiperiplanar conformation and the structure was optimised; giving an energy of -231.69253528 Hartree and a point group Ci/C1 (Which of the two point groups is it? João (talk) 09:23, 24 March 2015 (UTC)).
Then the gauche linkage of 1,5-hexadiene was drawn and optimised; giving an energy of -231.68302548 Hartree and a point group Cs/C1. Both these energies are very similar with the antiperiplanar conformer being slightly lower in energy, this is what is expected simply judging from sterics.
In figure 2 a lower energy gauche conformer can be seen, with an energy of -231.69266122 Hartree.
Then anti2 conformation was built and optimised giving an energy of -231.69253510 Hartree and a point group Ci - seen figure 3. The value given for the energy of this conformer is -231.69254 Hartree, which is the same as the value calculated from Gaussian (to less decimal places).
The anti2 conformer was then reoptimised at the B3LYP/6-31G* level giving an energy of -233.33634309 Hartrees. (How does the structure compare to the structure you had optimized before? João (talk) 09:23, 24 March 2015 (UTC))
A frequency calculation was then performed on the B3LYP/6-31G* optimised molecule. This confirmed that this is a minimum as all the vibrational frequencies were real and positive.
i) Sum of electronic and zero-point Energies= -233.192896 ii)Sum of electronic and thermal Energies= -233.185589 iii) Sum of electronic and thermal Enthalpies= -233.184644 iv) Sum of electronic and thermal Free Energies= -233.224571
Optimizing the "Chair" and "Boat" Transition Structures
The boat and chair transition structures of the cope rearrangement are made and optimised so they can be compared. To do this firstly a C3H5 allyl fragment is built and optimised to HF/3-21G level of theory. Then two of these optimised fragments were combined to make a rough guess of what the chair transition state should look like, setting the two fragmetns 2.2 Å apart.
allyl fragment, energy -115.82304010 Hartree, C2v point group
This guess was then optimised in two ways. The first was optimising it to a transition state by selecting optimization to a TS (Berny) in the calculate menu.This gave bond forming/bond breaking bond lengths 2.02159 Å and 2.02098 Å, and an energy of -231.61932181 Hartree. (Did you confirm the structure you obtained was a transition state? João (talk) 09:23, 24 March 2015 (UTC))
Chair, optimised to a TS
The next method of optimising the chair transition state was to optimise it using the frozen coordinate method. This involves freezing the allyl fragment bonds that will be made to a set length (2.2 Å) then optimising the structure to a minimum. Then unfreezing the bonds and optimising the structure to a transition state. This method gave bond forming/bond breaking bond lengths 2.02071 Å and 2.02074 Å, and an energy of -231.61932233 Hartree.
Chair, optimised with frozen coordinate method
These two method gave very similar results with the frozen coordinate method giving a slight lower energy and short bond lengths. (Did you confirm the structure you obtained was a transition state? João (talk) 09:23, 24 March 2015 (UTC))
The QST2 method was used to optimise the boat transition structure. For this two gauche conformers of 1,5-hexadiene were put into GaussView and numbered so they corresponded to the reactant and product of the cope rearrangement. Then the QST2 calculation was run. This gave bond forming/bond breaking bond lengths 2.14181 Å and 2.14046 Å, and energy -231.60280217 Hartree. This is a higher energy and longer bond lengths then the chair transition state, implying that the reaction would prefer to go via the chair transition state. Although, there's not much in it. (Did you compare with experimental values? Did you see how different levels of theory compare with experimental values? João (talk) 09:23, 24 March 2015 (UTC))
Optimised boat QST2 method
The Diels Alder Cycloaddition
GaussView was used to build cis butadiene and then optimized to the HF/3-21G level. The HOMO and LUMO were plotted and it can be seen that both orbitals are asymmetric with respect to the plane. (Indeed they are anti-symmetric with respect to the plane of the molecule, they are π orbitals. What other planes of symmetry are there? Would symmetry with respect to these be important to determine the outcome of the reaction? João (talk) 09:23, 24 March 2015 (UTC))
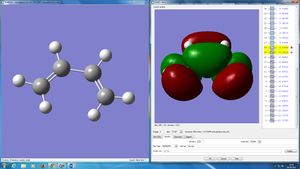
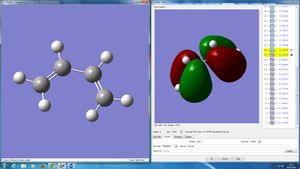
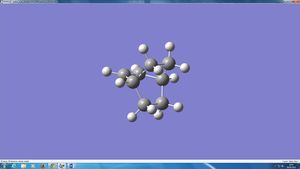
In order to obtain a transition state geometry of the reaction between ethylene and butadiene, firstly a bicyclo system was built in GaussView and optimised to a minimum. Then the CH2CH2 fragment was deleted and the transition state optimised using the frozen coordinate method, setting the interfragment distance originally as 2.2 Å. The transition state had transition bond lengths of 2.200130 Å, this is higher than the Van der Waal radi of carbon atoms (around 1.75 Å1) - meaning that the bond has not formed. (However, the bosd distance is inferior to twice the van der Waals radius for carbon. Is that significant? João (talk) 09:23, 24 March 2015 (UTC))


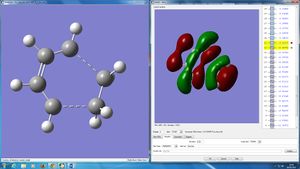
The HOMO of this transition state can be seen to be symmetrical whilst the LUMO is asymmetrical. (How do these relate to the orbitals of the reactants? João (talk) 09:23, 24 March 2015 (UTC))
The Diels-Alder reaction between cyclohexa-1,3-diene and maleic anhydride can potentially give either an endo or an exo product. To examine which would be favoured we can examine the exo and endo transition states, to see which is lower in energy. First, cyclohexa-1,3-diene and maleic anhydride are built and optimised to the HF/3-21G level. Then these are used to build a rough estimate of the endo and exo transition states. Primarily the endo adduct is given as this is lower in energy (and this reaction is kinetically controlled.

References
1. R. Scott Rowland, Robin Taylor: Intermolecular Nonbonded Contact Distances in Organic Crystal Structures: Comparison with Distances Expected from van der Waals Radii. In: J. Phys. Chem. 1996, 100, S. 7384–7391, doi:10.1021/jp953141+.
