User:Bw08
Module 1: Organic
The Value of Computational Chemistry
Computational chemistry is a multidisciplinary area of science transcending traditional barriers sparating biology, chemistry, physics and mathematics. As theory can help to rationalize experimental observation, provide information not amenable to experimentation and even make predictions concerning the outcome of future experiments, it has become widely accepted by experimental scientists as a valuable supplement to their existing studies. One aspect of applied theory involves molecular simulations at the atomic level, which is sometimes called “molecular modeling”.
Molecular Modeling
Two broad categories of chemical modeling may be considered: macroscopic models and microscopic models. Macroscopic models consider, for example, rates of uptake of material and kinetics of transport, without consideration of structural features of the individual molecules. In contrast, microscopic models (often called atomistic models) usually take full account all the atoms in the system.
Microscopic, atomistic modelling is done in two ways: using fitting procedures or by applying theory from first principle. The fitting procedures are an attempt to rationalize connections between molecular structure and physiochemical properties (quantitative structure property relationships, QSPR) or with biological response (quantitative structure activity relationships). This type of modelling requires use of existing data to create the model and then allows interpolation or extrapolation of new properties of as yet unknown molecules within that same class of compounds.
The second kind of atomistic modelling applies theory, usually from first principles. Three commonly used modelling techniques involve the use of quantum mechanics, molecular mechanics or molecular dynamics. During the first part of this investigation, molecular mechanics was used to carry out the calculations.
Equations etc.
Notable Limitations of the Molecular Mechanics Approach
Firstly, it is an essentially parametric method, using data from well characterised molecules. Hence this technique is interpolative rather than extrapolative, hence it cannot deviate far from commonly observed chemistry. This results in difficulty in assessing the kinetic control of a reaction using the standard approach, as that requires parameters of the transition state. It is also challenging to model new molecules with unusual bonding, for these reasons mentioned, therefore complete quantum mechanical treatment of the system is necessary. Molecular orbital theory, another quantum mechanical method, may also have to be turned to for analysis of molecular properties such as stereoelectronic effects, aromaticity, hyperconjugation and frontier orbital interactions. Often molecular mechanics parameters are available for only certain bond types, and are unavailable for many functional groups. Metal ions are also more difficult to handle using this type of model.
Hydrogenation of Cyclopentadiene Dimer
Dimerisation of Cyclopentadiene
At room temperature, cyclopentadiene will slowly react with itself to form dicyclopentadiene. This reaction is an example of a [4π+2π] cycloaddition (when referring to the number of atoms in the system), or a [3+2] cycloaddition (when referring to the number of atoms involved). This reaction proceeds via a Huckel transition state (4n+2) with suprafacial topology.
There is a high degree of stereospecificity in this reaction. Two possible compounds can be formed, the endo- or exo- product. The overall reaction is shown below.
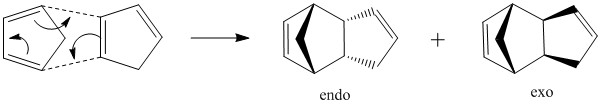
The total energy of each product was computed as is shown in the table below, alongside the individual contributions.
| Energy parameter | Endo energy (kcal mol-1) | Exo energy (kcal mol-1) |
|---|---|---|
| Stretch | 1.255 | 1.281 |
| Bend | 20.826 | 20.570 |
| Stretch-Bend | -0.834 | -0.837 |
| Torsion | 9.512 | 7.667 |
| Non-1,4 VDW | -1.499 | -1.415 |
| 1,4 VDW | 4.310 | 4.236 |
| Dipole/Dipole | 0.445 | 0.378 |
| Total energy | 34.0136 | 31.8796 |
This indicates that the endo dimer has an energy which is ~2kcal mol-1 higher than the exo dimer. This is consistent with the ' endo rule' for Diels Alder reactions. In general the two hydrogen atoms briding the two cyclic structures must be cis in both products, but there are two possible products which obey this rule. These are called the endo and exo products. Although the endo product is higher in energy, it is produced under these kinetically controlled conditions, as the transition state leading to this product is stabilised relative to that leading to the exo product. This is represented in the diagram below.
Another way of looking at this result is by recognizing the negative entropy of activation as two molecules combine to produce one. A very precise orbital orientation is needed for the two bonds to form at once, which is assisted by the through space HOMO/LUMO bonding interaction.
Hydrogenation of Endo Dimer
Hydrogenation of the endo cyclopentadiene tends to give a dihydro derivative; a tetrahydro derivative is only formed under prolonged hydrogenation conditions.
By analysing the energies of the two dihydro derivatives of the endo dimer this enables an informed prediction as to which C-C double bond will be hydrogenated first.
| Type of Energy | Molecule 3 /kcal mol-1 | Molecule 4 /kcal mol-1 | Energy Difference /kcal mol-1 |
| Stretch | 1.24 | 1.10 | 0.14 |
| Bend | 18.97 | 14.51 | 4.46 |
| Stretch-Bend | -0.76 | -0.55 | -0.21 |
| Torsion | 12.15 | 12.50 | -0.35 |
| Non-1,4 VDW | -1.56 | -1.51 | -0.05 |
| 1,4 VDW | 5.73 | 4.51 | 1.22 |
| Dipole/dipole | 0.16 | 0.14 | 0.02 |
| Total Energy | 35.93 | 31.51 | 4.42 |
Hydrogenation product 4 is more stable than compound 3 by about 4 kcal mol-1), suggesting that the facility of hydrogenation is greater when compound 3 is formed. This prediction is made purely on a thermodynamic analysis of both compounds. This suggests that the double bond in the bridged ring is easier to hydrogenate than the unbridged double bond. Each compound has similar contributions to the total energy from the stretching, torsion, Van der Waals and hydrogen bonding energy terms, whilst the largest difference is observed between the bending contributions ( 4.5 kcal mol-1).
The bending energy of molecule 3 is much higher than in molecule 4, showing that the structure of molecule 3 is relatively unstable with a large deviation of bond angles from the ideal molecule. Furthermore, the C=C bond is found closer to the tertiary carbon atom in molecule 3 than in molecule 4. This shows that there is an increase in steric hindrance and strain in molecule 3. Therefore, the rotation of the bond is restricted and the increase in bending would lead to the relief of strain in the norbornene.
Finally, a comparison is made between the H-C=C and C-C=C bond angles found in both molecules as it is closely linked to the points outlined above. The ideal angle for a sp2 hybridized carbon is 120°. Consider the H-C=C bond angles in molecules 3 and 4, which are 108° and 113° respectively. The greater reduction of H-C=C bond angle in compound 3 indicates a higher level of strain relative to compound 4, as it is the furthest from the ideal value of 120°.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
Part (a)
There has been widespread interest in the regio- and enantioselective conversion of 2-substituted nicotinic acid derivatives A into C(4) substituted dihydropyridines B and other compounds. Consequently, synthetic intermediates of type B should be of use in the enantioselective construction of alkaloids containing the piperidine ring system.
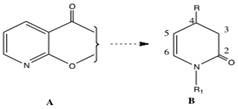
A large number of studies concerned with the addition of carbon nucleophiles to pyridines have been reported. Both the “free base” and the activated pyridinium salts undergo 1,2-, 1,4- and 1-6 additions.
The first reaction investigated in this area involves the optically active derivative of prolinol (5) which undergoes a nucleophilic addition with methyl magnesium iodide to alkylate the pyridine ring in the 4-position, with the absolute stereochemistry shown in 6. Heterocycle 5 is prepared in 86% yield from 2-chloronicotinic acid and L-prolinol. It is also noteworthy that the alkylithium reagents are nearly regio- and stereo- random. The high regio- and stereo- selectivity of this reaction has been proposed to result from initial coordination between the Grignard reagent and the amide oxygen atom, as shown below.

Conjugate delivery of the methyl group from magnesium to C(4) of the pyridine ring generates a magnesium enolate, which then goes on to produce 6.
Dreiding stereomodels indicate that there is almost no flexibility in the seven membered ring of 5. Two conformational minima were found to be possible. One of these has the amide carbonyl group above the plane of the pyridine ring anti to the hydrogen atom at the chiral centre; the second has the carbonyl group and pyridine ring coplanar. Most importantly, no conformation was found to exist which positions the amide carbonyl group below the plane of the pyridine ring.
Using this information, the dihedral angle between the bonds highlighted in 5 above was varied in order to obtain the lowest energy. Initially the MM2 force field was used and the dihedral angle was tested for 0°, 5°, 10° and 20°. This clearly indicated the preferred conformation has a dihedral angle of about 10°, but complete analysis was carried out from 0-180° degrees giving the results shown in the graph below.
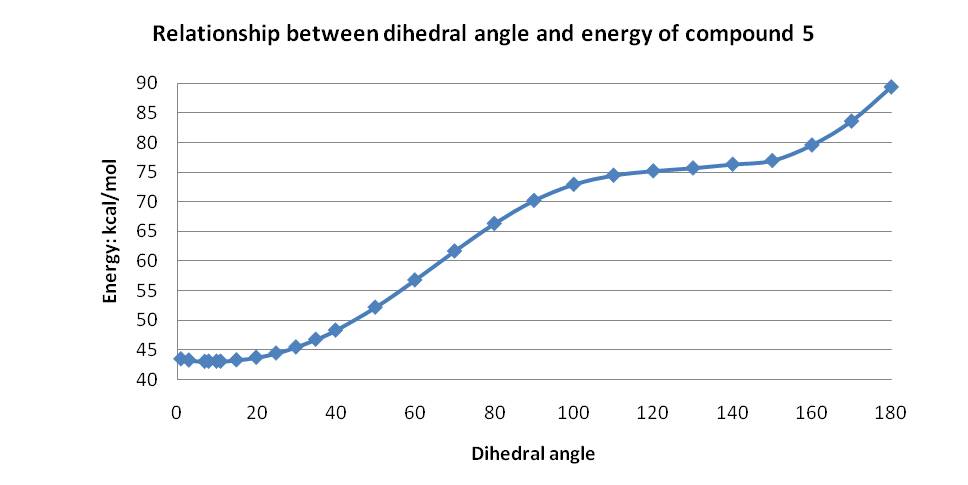
Minor products resulting from Grignard reactions of this kind and random addition products isolated when alkyllithium reagents are used are expected to derive from pathways not involving the type of chelation control shown in 5a.
Even higher selectivity and yield in this reaction type is expected and has been reported when bulkier reagents such as PhMgBr is used, promoting addition of the alkyl or aryl group anti to the hydrogen atom at the chiral centre.
Part (b)
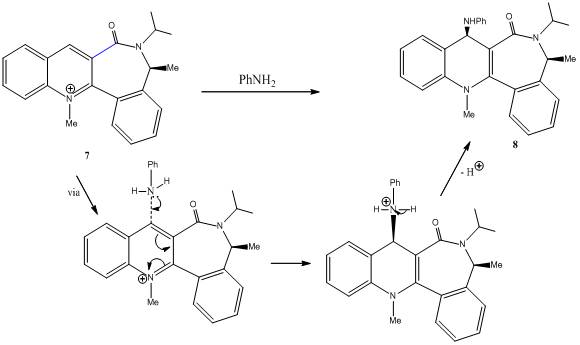
Several new synthetic tools have been developed from investigation of potential biomimetic NAD+ models as ‘nucleophile-transferring agents’. Compound 7 is an example of an axially chiral quinolinium salt. Nucleophilic addition of phenylamine to 7 yields 8 as the single diastereoisomer. The high level of selectivity is also observed when benzylamine or piperidine is used as the addition reagent. 2D-NOESY experiements, showing dipolar coupling between the methyl group of the lactam moiety and the methylene protons adjacent to the nitrogen of the amine confirm that both amines react on the opposite face with respect to the C=O lactam.
Using this information, MM2 analysis of the dihedral angle was carried out. The lowest energy was expected to be recorded when the C=O bond is below the plane of the pyridinium ring (negative dihedral angle). For completeness, energies of the conformations having the C=O bond above the plane of the ring were also computed.

This shows a minimum energy conformation when the dihedral angle is approximately -20°.
INSERT FIGURE
The excellent diasterofacial selectivity observed orginiates from steric control exerted by the carbonyl lactam. The results compiled by Leleu et al. suggest that the chiral axis defined by the C3-C=O bond (highlighted in blue in compound 7) is the main configurational element governing the stereoselectivity of this nucleophilic addition. This element plays a key role in terms of diastereoselectivity, during the ‘anchoring step’ of the amine on compound 7, in which case the methyl group attached to the chiral carbon acts as the ‘chiral inducer’.
The incoming nucleophile (phenylamine is this case) therefore reacts on the opposite face (anti) to the C=O lactam, giving the product 8.
| Jmol | - | <jmol>
<jmolAppletButton> <uploadedFileContents>untitled-2.mol</uploadedFileContents> <text>Conformer 5b</text> <color>#FFFFFF</color> <size>600</size> <script>spin on</script> </jmolAppletButton> |
| Jmol | - | <jmol>
<jmolAppletButton> <uploadedFileContents>untitled-2.mol</uploadedFileContents> <text>Conformer 5b</text> <color>#FFFFFF</color> <size>600</size> <script>spin on</script> </jmolAppletButton> |
Conclusion
One example of the extending reach of computational chemistry towards organic applications is in the area of cyclodextrins. Cyclodextrins are a family of compounds made up of sugar molecules bound together in a ring (cyclic olgosaccharides).
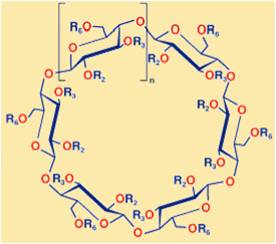
These compounds are able to form host-guest complexes with hydrophobic molecules given the unique nature imparted by their structure. As a result, these molecules have found a number of uses in a wide range of fields such as pharmaceutical applications for drug release.
Use of computational chemistry in this area has until recently been limited, due to the fact that they are relatively large, flexible molecules that are often studied experimentally in aqueous environments. This forces one to introduce many assumptions and restrictions so as to become unrealistic, creating a major hurdle for computational chemists. Many internal degrees of freedom exist for these molecules, making structural predictions both time consuming and difficult. Nevertheless, there have been a significant number of computational studies in the past decade that have led to a more profound understanding of the structure, dynamics and chemical behaviour of these compounds.
References
[1].
Literature
- ↑ 1.0 1.1 The Ultrafast Photoisomerizations of Rhodopsin and Bathorhodopsin Are Modulated by Bond Length Alternation and HOOP Driven Electronic Effects DOI:10.1021/ja1056196
