User:Boultwoodtom
Module One - The basic techniques of molecular mechanics and semi-empirical molecular orbital methods for structural and spectroscopic evaluations
Objectives
Computational based programs now allow us to accurately model organic structures and predict their reactivity, whilst rationalising the outcome of the reaction. Computational based programs can also enhance synthetic experiments by suggesting improvements to reaction schemes based on energetics of transition states and final products.
To use molecular mechanics to predict the geometry and regioselectivity of:
- the hydrogenation of cyclopentadiene dimer
- the stereochemistry of nucleophilic addition to two different pyridinium NAD+ analogues.
- the conformation/atropisomerism of a large ring ketone intermediate in one synthesis of the anti-cancer drug Taxol
To use semi-empirical and DFT molecular orbital theory to investigate:
- the origins of the regioselectivity of the electrophilic carbenylation of a chloro-substituted bicyclic diene,
- the use of DFT molecular orbital theory to investigate Neighbouring group participation (NGP) on the C-Cl and/or C=C stretching frequency of the above bicyclic diene
- investigation of spectroscopic simulation in 1-bromo-2-phenyl-2-propanol.
The Hydrogenation of Cyclopentadiene Dimer
This dimerisation reaction is characteristic of a Diels-Alder reaction, which can be considered as a concerted [4+2] cycloaddition involving the π based electrons from a diene and dienophile[1]. Stereochemical features of the Diels-Alder reaction name exo and endo systems are observed when there is suitable substitution on the diene and the dienophile. This is because the reactants can approach each other from two different faces. This can either result in the substituent on the dienophile being directed away from the diene (exo substitution) or the dienophile being directed towards the diene (endo substitution). In most Diels-Alder reactions, when the product distribution is under kinetic control, the endo adduct is preferentially, sometimes exclusively, formed. This is due to a number of stereochemical and stereoelectronic reasons.
- Endo addition is a result of "maximum accumulation of double bonds"[2] between the diene and the dienophile when the two starting materials are in the same orientation. This affords the most stable transition state, with the most stable transition state leading to the major product.
- Orbital symmetry considerations
- The transition state can be described using several resonance structures. One of this resonance structure is a zwitterion. In the endo transition state, the proximity of the two charges stabilizes this structure and thus favours the endo addition.[3]
Using Chem3D, the possible cyclopentadiene dimers and their hydrogenation products were gemometrically optimised using the MM2 forcefield option, with the relative stabilities of the pairs of compounds 1/2 and 3/4 (shown below) indicating which of each pair is the less strained and/or hindered in a thermodynamic sense.

| Molecule | Energy (kcal/mol) |
|---|---|
1 |
31.8860
|
2 |
34.0000
|
3 |
35.6895
|
4 |
31.1613
|
From the energies in the above table from the geometric optimisation, it is clear to see that Product 1 from the Diels-Alder dimerisation reaction is the lowest in energy and therefore under thermodynamic control, resulting in the exo-product with both hydrogens bending away from the plane of the rings which is thermodynamically favoured according to the calcuations performed. With dimerisation product 2 being higher in energy and the hydrogens from the ring fusion pointing towards each other, this product can be assigned as the endo product under kinetic control.

In the two hydrogenation products 3/4, it can be observed that product 4 is of lowest energy and therefore thermodynamically more stable than product 3. Both of these products originate from the kinetic product 2, with the more thermodynamically stable product 1 not being formed. This is consistent with the "endo addition rule" as described earlier, with endo selectivity being the basis of the major product. The stability of the isomers is depednant on which of the double bonds is hydrogenated. The relative contributions from the stretching (str), bending (bnd),torsion (tor), van der Waals (vdw) and hydrogen bonding (H-Bond) energy terms in terms can be compared to analyse the relative stability of 3 and 4. Each term indicates the deviation from "normality" of the particular function. A very positive stretch term would indicate the predicted bonds are far from the "natural" lengths, due to some geometrical feature of the molecule.
Molecule |
Stetching (str) |
Bending (bnd) |
Torsion (tor) |
van der Waals (vdw)
|
|---|---|---|---|---|
3 |
1.2709 |
19.8595 |
10.8238 |
5.6392
|
4 |
1.1006 |
14.5335 |
12.5094 |
4.4961
|
Product 4 is shown to be thermodynamically more stable due to the overall energy of the conformation, whereas the formation of product 3 can be due down to kinetic control. Comparing the relative contributions it is clear to see that the main difference between Molecule 3 and Molecule 4 are the bending contributions. Molecule 3 is far from the natural lengths and orientation compared to Molecule 4 which should result in a higher energy conformation which is shown in the MM2 calculations above. The main cause of this is decreased bond angles in Molecule 3 forcing the substituents closer together increasing steric repulsion effects.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue).

The first synthetic scheme involves the optically active derivative of prolinol, 5, which reacts with methyl magnesium iodide to alkylate the pyridine ring in the 4-position, with the absolute stereochemistry shown in 6 to produce pyridoxazepinone. The alkylating agent is a simple Grignard reagent, MeMgI - methyl magnesium iodide. N-methyl salts of nictinic acid undergo regioselective addition with Grignard reagents to give the major product denoted in 6. As the Grignard reagent becomes more sterically hindered, the regioselectivity of the transformation is enhnaced so that only Product 6 is synthesised. Co-ordination between the Grignard and the amide oxygen allows conjugate delivery of the organic ligand from Mg to the carbon of the pyridine ring generating the magnesium enolate. The delivery of the Me group is orientated anti to the H atom at the chiral centre of the optically active prolinol auxillary.
There is little flexibility in the 7 membered ring adjacent to the pyridine group within the molecule. This has a major effect in the conformations available for the alkylation via the Grignard reagent to occur. There are only two possible conformational minima possible.
- The amide carbonyl group is ABOVE the plane of the pyridine ring, anti to the H atom at the chiral centre
- The other conformer has the amide carbonyl group and pyridine ring coplanar.
The conformation in which the amide carbonyl group being BELOW the ring is not possible.
Using ChemBio3D once again, the stuctures of the reactant and the product were modeled and the energy minimized. This time the models were minimized using the MMFF94 force field, with the results shown below:
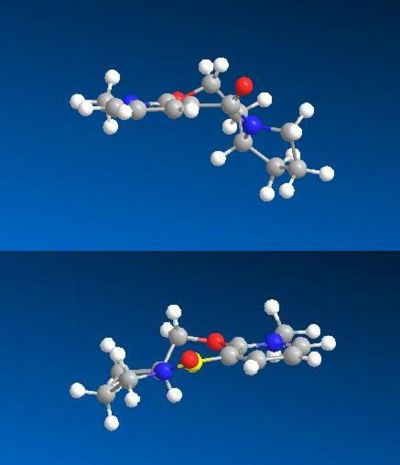
Due to the known chelating effect of the magnesium ion in the Grignard reagent with the oxygen in the amide carbonyl, the predominant compound formed is the one with which the carbonyl is pointing upwards. This can be deduced by the stereochemistry of the final product, 6, as the methyl group has been delivered to the top face of the conformer. The energies of the two systems are shown below and are very similar in energy. The confromation with the C=O up is shown to be slightly higher in energy, but is stereochemically proven to be the confromation which leads to the product. Therefore one can assume that this reaction is under kinetic control.
Molecule |
Energy (kcal/mol)
|
|---|---|
5 (up) |
59.1658
|
5 (planar) |
57.5376
|
6 |
-1.5282
|
In the second example, the pyridinium ring of 7 is derivatized by reaction with aniline to form 8 which acts to transfer the NHPhenyl group to electrophiles (e.g. carbonyl groups) in a reverse of the reaction by which it was formed. In this reaction, 1,4 adducts are formed with respect to the carbonyl species with the amine acting as a masked nucleophile. The masked nucleophile reacts with an electrophile (such as the C=C present in the nitrogen heterocycle, adjacent to the carbonyl). The amine reacts on the opposite face with respect to the carbonyl lactam. This diastereofacial selectivity originates from steric control exerted by the carbonyl lactam.
The energies were once again minimised using the MMFF94 force field, with the results shown below:
Molecule |
Energy (kcal/mol)
|
|---|---|
7 |
99.4823
|
8 |
46.4411
|
As seen in the modelling image of compound 7, it is evident that the amide carbonyl group is pointing downwards with respect to the pyridine ring system. This blocks the bottom face of the molecule off from nucleophilic attack and induces the large sterically hindered amine to attack from the top face which gives the stereochemistry shown. This is opposite to the the example shown with compound 5, where the carbonyl lactam chelates with the Grignard reagent forcing the stereochemistry to which way the carbonyl was in the ring system, which was up compared to the pyridine ring system. Both of these models only consdier the energies caclulated by the MMFF94 forcefield, which allow us to assess which of the conformers are most likely to lead to the desired product through steric interactions with the incoming nucleophile. However, the calculations do not allow us to assess the orbital interaction between the incoming nucleophile and the lactam carbonyl due to the restrictions of the molecular modelling methodolgy. If we considered the HOMO/LUMO MO picture for the carbonyl lactam, the definitive stereochimsitry of the product could be more accurately depicted.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
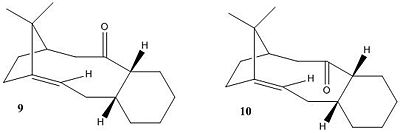
Shown are key intermediates (9 and 10) in the synthesis of Taxol which is an important drug in the treatment of ovarian cancers. The carbonyl group in these intermediates can either be pointing up or down and are able to interconvert via isomerisation reactions. Carbonyl addition reactions to these intermediates depend on the stability of each of these isomers. This kind of behavoiur can be described as atropisomerism. Atropisomerism can be defined as the distinction between two molecules that arises due to restricted rotation about a single bond due to high steric demand, removing the capabilities of a freely rotating system. This effect is usually found in susbsituted aromatic rings within macrocyclic structures.
Using ChemBio3D and molecualr mechanics MM2 force-field, the energies of the two different carbonyl systems were minimised to determine the most stable isomer and determine why alkene functionalisation reactions occur slowly. On orginal assessment of the energies of the systems, there were large deviances in the results being obtained so further optimisation had to take place. The original conformations of the structures were improved by further minimisations by placing the cyclohexane rings in their appropiate lowest energy chair conformations, from their original boat conformations. This has the effect of lowering the overall energy of the system. MMFF94 calculations were also undertaken for comparitive reasons with the molecular mechanics MM2 force field calcualtions and all results are shown below:
From both minimisations it is evident that intermediate 10 (C=O down) is the most stable intermediate and this is proven by both minimisations resulting in lower energies compared to conformer 9 (C=O up). From the relative stabilisation of the of the two interconverting forms, a relative stabilisation between the boat and chair forms of cyclohexane can be estimated at around 5 kcal/mol stability in favour of the chair conformer. The alteration of boat to chair conformer resulted in a lowering of the overall energy of the system both in the MM2 and MMFF94 cases by approximately the same energy. Pawar et. al. quote this energy to be around 5.5 kcal/mol[4], which is in good agreement with this experimental data.
Hyperstability and Rates of Hydrogenation
Hyperstability is characterized by alkenes and their reluctance to by hydrogenated even under drastic catalytic conditions[5]. Hyperstability was a concept introduced in 1981 by P.v. R Schelger et. al. which considers the relative strain energy of a cycloalkene (e.g. cyclohexene) and its corresponding cycloalkane (e.g. cyclohexane)[6]. It is noted that the strain energy of cycloalkenes are higher than that of the corresponding cycloalkane, giving cyclohexene a positive Olefin Strain Energy (OSE). The work of Schelger showed some inconsistencies whereby several cycloalkenes showed negative OSE values. This indicated that the strain energy in the cycloalkane product was larger than expected. This was put down to vicinal and trans annualar H-H interactions. These cycloalkenes are deemed to be hyperstable. This hyperstability has also been proved by S. W. Elmore and L. A. Paquette in their studies of thermally-induced retro oxy-Cope rearrangements[7]. In both of the Taxinol intermediates, a bridgehead double bond is present which is formally forbidden by Bredt's Rule due to the associated strain of the molecule. In comparison of both C=O derivatives, the MM2 and MMFF94 calculations have proven to show that the conformation with C=O pointing downwards is lower in energy. Considering the hydrogenated derivative, a 10 membered cycloalkane ring is formed. The formation of 8, 9 and 10 cycloalkane rings is highly unfavourable due to trans annnular strain caused by unfavourable H-H interactions. This gives the alkene derivative a degree of stability over its unfavourable hydrogenated product, resulting in the extremely slow hydrogenation of the alkene product. A JMOL application of the hydrogenated product is shown below showing the transannular strain.

Comparison of MM2energies can't be considered as they are only useful for considering stereoisomers. However, the heat of formation of both can be comapred using the MOPAC methodolgy. The MOPAC method calculates the heat of formation for the alkene derivative to be 65.07kcal/mol compared to that of the dihydro derivative 60.13kcal/mol. This gives a rough estimation to the stability of both compounds relative to each other.
Modelling Using Semi-Empirical Molecular Orbital Theory
In part 1, the strengths and weaknesses of a purely mechanical molecular model were illustrated. In particular, the endo stereoselectivity in Diels Alder cycloadditions was attributed to "secondary orbital" interactions, which the Molecular Mechanics approach cannot handle. In this section, such electronic aspects of reactivity will be illustrated, showing how explicit consideration of the electrons in molecules must be taken into account, and how the electrons influence bonds and derived spectroscopic properties.
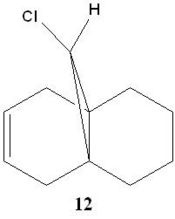
Regioselective Addition of Dichlorocarbene

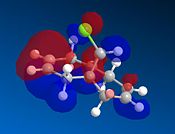
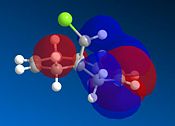
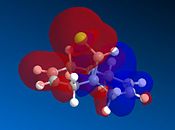
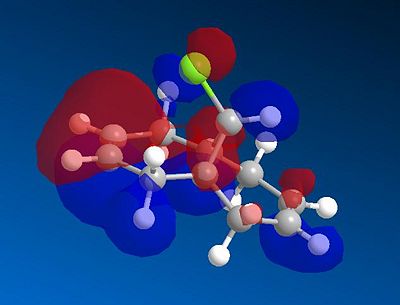
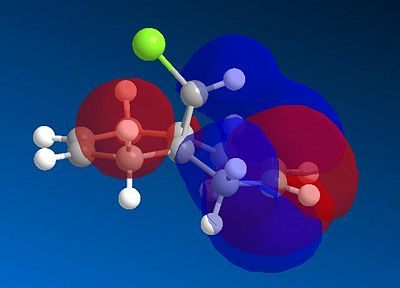
This experiment will illustrate the transition from a purley classical mechanical treatment of a molecule to a quantum mechanical treatment. This QM description includes the wave-description of the electrons. Orbital control is an important consideration when considering the regioselectivity of electrophilic additions to reagents such as that shown by molecule 11. The geometry of the molecule is predicted, energy minimised and the orbitals displayed graphically. This is done using the ChemBio3D software using the MOPAC/PMC method which provides an approximate representation of the valence-electron molecular wavefunction. The HOMO-1, HOMO, LUMO, LUMO+1 and LUMO+2 molecular orbitals can be modelled using this approximation. The HOMO is considered to be the most reactive towards electrophilic attack and the LUMO the most susceptible to nucleophilic attack. The graphical representations of the 5 molecular orbitals are shown below and can be used to identify which alkene is more nucleophilic due to the molecular orbitals based on them.
In rigid systems free from steric control, regioselectivity can arise from oribtal and electrostatic control. An electrophile would generally attack the HOMO due to the presence of the highest energy electrons in the system, which are generally the most reactive and most accessible. It can be seen that for the HOMO MO surface diagram (enlarged below), the MO's are predominatley based on the alkene syn to the chlorine. This indicates that the electons in the HOMO have the greatest probability of being located in this alkene, and is therefore more susceptible to electrophilic attack. As with regards to the alkene anti to the chlorine atom, the MO wavefucntion is very small meaning that a small probability of electron occupation reducing its nucleophilicity. This analysis can also be used with the LUMO (Lowest Unoccupied Moelcular Orbital). The LUMO is higer in energy than the HOMO and is suscpetible to nucleophilic attack. The MO surface diagram shows a larger MO surface over the alkene anti to the chlorine. This is good agreement with the analysis provided by the HOMO as it shows that the alkene anti to the chlorine has greater electrophilic character and hence is more susceptible to nucleophilic attack. The reactivity of the double bonds can also be associated with stabilising interactions within the molecule between suitable MO's. Stabilising antiperiplanar interactions between Cl-C σ* orbital and the occupied C=C anti to Cl atom makes the C=C bond syn to the Cl atom more nucleophilic. in both frontier orbital and electrostatic control.[8]
| HOMO-1 | HOMO | LUMO | LUMO+1 | LUMO+2 |
|---|---|---|---|---|
 |
 |
 |
 |
 |
The two moelcules will now be comapred to assess the effect of the C-Cl bond on the stretching frequencies of the molecule. Molecule 11 and its dihydro derviative (Molecule 12), where the double bond anti to the chlorine atom has been hydrogenated will be compared. Gaussian can be used to calculate the stretching modes and frequencies of both molecules, and from here produce IR spectra. Both IR spectra of the dihydro derivative and the dialkene have been produced and are shown below. These will be compared to assess the effect of the C-Cl bond on both spectra:

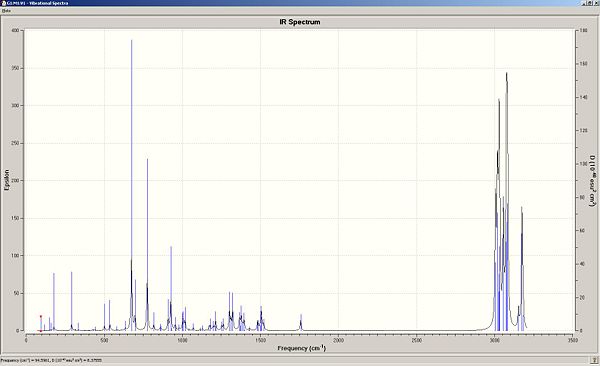
| 11 (dialkene) | Stretching frequency (1/cm) | Intensity | Dipole strength |
|---|---|---|---|
C-Cl |
770.81 |
~ 150 |
130.087
|
C=C (syn to Cl) |
1757.43 |
~ 15 |
8.953
|
C=C (anti to Cl) |
1736.99 |
~ 15 |
9.620
|
12 (dihydro) |
Stretching frequency (1/cm) |
Intensity | Dipole strength |
C-Cl |
774.97 |
~ 230 |
103.118
|
C=C (syn to Cl) |
1758.06 |
~ 25 |
9.831
|
C-C (anti to Cl) |
1398.47 |
~ 5 |
0.429
|
The IR spectra show the vibrational frequencies, epilsom values (related to intensity via Beers Law) and dipole strength values. The ta
ble above shows the varying stretching frequencies of the alkene stretches and the C-Cl stretch in Molecule 11 and Molecule 12. In Molecule 11, the C=C stretches have been identified and can be assigned to the double bond anti to the Cl atom and the double bond syn to the Cl atom. The C=C syn to the Cl atom has a reported stretching frequency of 1757.43cm-1 and the C=C anti to the Cl atom is reported as 1736.99cm-1. In comparison to the dihydro derivative, Molecule 12, only the syn C=C can be consdiered as the anti C=C has been hydrogenated. The syn C=C stretching frequency occurs at 1758.06cm-1 which is of a very similar frequency to that of its precursor. It can be noted that the resulting single C-C bond from hydrogenation has a much lower stretching frequency compared to its parent alkene of 1398.47cm-1. This is expected due to the strength of the bonds. As the bond strength increases, more energy is required to stretch it. As discussed previously, the stabilizing interaction of the the Cl-C σ* orbital with the anti C=C pi oribtal causes a weakening of the C-Cl bond as there is more electron denisty in the anti-bonding system. As the anti C=C bond donates electron density to the C-Cl antibonding orbital, there is an overall reduction in bond order of the alkene. This is reflected in the vibrational freqquencies of the anti C=C and the syn C=C. A reduction in bond order would effectively make the bond weaker, reducing the vibrational frequency compared to the syn C=C (1736.99 cm-1 vs 1757.43cm-1). In the hydrogenated derivative, Molecule 11, the anti- C=C bond is no longer present so I would expect the interaction between the pi bonding oribtals of the alkene with the C-Cl sigma * to be absent. This theoretically should mean that the C-Cl bond strength is higher than that of its alkene precursor due to no donation of eelctron density into the antibondng orbitals. This is seen in the calculations (770.81 cm-1 for Molecule 11, 774.97cm-1 for Molecule 12).
Investigating the effect of adding an EWG (Electron Withdrawing Group) to alkene system
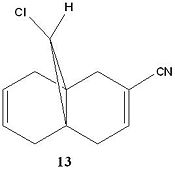
The -CN ligand in organic synthesis is well known to be a highly electron withdrawing group due to its ability to accept electron density into low lying available orbitals. As before with the dichlorocarbene precursor, the same quantum mechanical treatement of the molecule was taken with geometry optimised using MM2 and MOPAC parameters and the IR spectrum predicted using Gaussview. This will allow us to understand the effect of the EWG on the C-Cl and both alkene stretching frequencies. The resulting IR spectrum and predicted stretching frequencies are shown below:
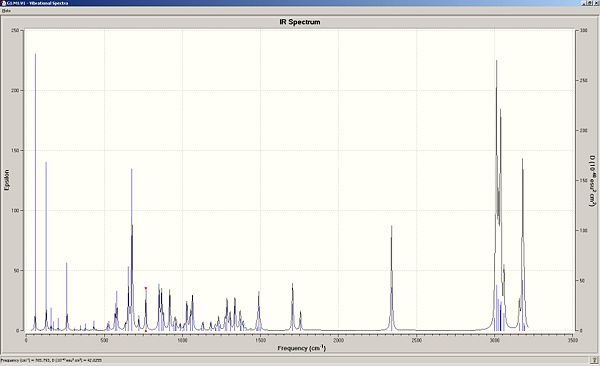
| Molecule 13 (CN derivative) | Stretching frequency (1/cm) | Intensity | Dipole strength |
|---|---|---|---|
C-Cl |
765.29 |
~ 35 |
42.0255
|
C=C (syn to Cl) |
1756.54 |
~ 40 |
10.9566
|
C=C (anti to Cl) |
1706.31 |
~ 20 |
26.7206
|
It is evident that the attachment of the CN ligand to the anti C=C has had a great on the stretching frequency of the anti C=C bond compared to its dialkene precursor where R = H instead of R = CN.
Structure based Mini Project using DFT-based Molecular Orbtial methods
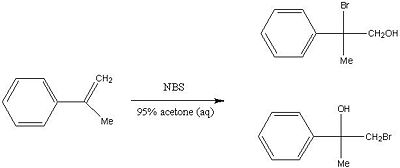
In this structure based mini project, I will be assessing the preparation of the bromohydrin of α-methylstyrene. Bromohydrin formation reactions are ideal for studying the regioselectivity of electrophilic addition reactions because two dissimilar groups are added across the π bond.
Mechanistic Considerations through Markovnikov's rules
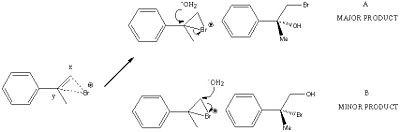
The major product is the regioisomeric reaction can be predicted by using Saytzev's rules. As shown in the mechanism below, the formation of the bromonium ion by the electrophilic attack of Br2 across the styrene double bond results in two different possilbities for attack by a nucleophile, x and y. We can consider the formation of the bromonium ion as NBS can be considered as a source of "cationic" bromine[9]. Nucleophilic attack will occur by the water molecule to the most hindered end of the bromonium ion in an SN2 fashion, giving the most stable and most substituted carbocation (hyperconjugation), which is most able to support positive charge in accordance with Markovnikov's rule rules[10]. The least favoured product will be that where the nucleophile attacks and the least substituted carbocation is formed, x. A simple proton transfer gives both isomers, A and B.
Spectroscopic Considerations Using Computational Methods
In this reaction, two different regioisomers are formed and can be separated by spectroscopic methods such as 13C NMR. Computational methods will be used to predict the 13C NMR and results will be comapred to literature reports. 1 H NMR will also be predicted along with IR spectroscopy data. The energy of both isomers were minimized using MM2, resulting in isomer B being optimized to an energy slightly more than that of isomer A (6.0034 kcal/mol vs. 6.6361 kcal/mol). Both of these isoemrs were then geometrically optimized using DFT = MPW1PW91 with a basis set of 6-31G(d,p). The geometrically optimized structures were then submitted for NMR prediction, with CDCL3 as solvent, with the results and analysis shown below. The NMR spectra are referenced to TMS MPW1PW91/6-31G(d,p) CDCl3 GIAO. It must be noted that carbons attached to heavy elements must have their shifts corrected. In the product isomers, a C-Br bond is present which is corrected accordingly by -12ppm to allow for spin-orbit coupling errors.
| 13C NMR Spectrum Isomer A | 13C NMR Spectrum Isomer B | |
|---|---|---|
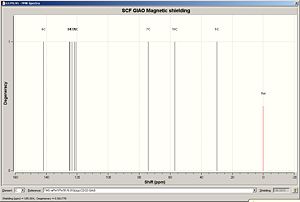 |
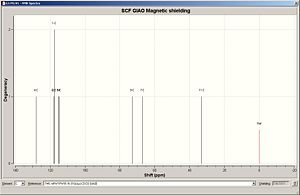 |
| Assignment | Chemical shift / ppm | Corrected chemical shift / ppm | Literature chemical shift / ppm |
|---|---|---|---|
C1 (para) |
123.41 |
||
C2 (meta) |
124.86 |
||
C3 (ortho) |
121.68 |
||
C4 (ipso) |
141.78 |
||
C5 (ortho) |
120.71 |
||
C6 (meta) |
124.72 |
||
C7 (tetrahedral centre) |
74.15 |
79.2
| |
C9 (methyl centre) |
30.06 |
28.4
| |
C10 (methylene, Br bearing) |
57.03 |
45.03 |
48.5
|
| Assignment | Chemical shift / ppm | Corrected chemical shift / ppm | Literature chemical shift / ppm |
|---|---|---|---|
C1 (para) |
117.6 |
||
C2 (meta) |
117.7 |
||
C3 (ortho) |
115.0 |
||
C4 (ipso) |
128.1 |
||
C5 (ortho) |
114.9 |
||
C6 (meta) |
118.0 |
||
C7 (tetrahedral centre) |
67.1 |
55.1 |
49.7
|
C9 (methylene, OH bearing) |
72.9 |
79.5
| |
C11 (methyl centre) |
33.0 |
26.9
|
| Isomer A Assigment | Isomer B Assigment |
|---|---|
 |
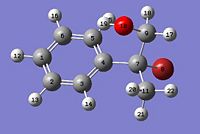 |
|
|
Literature values for the benzene carbons was unavialble in the literature data and only the tetrahedral sp3 carbon can be compared to literature data. Results for the experiental 13C NMR are compared to the literature data above. The first thing to notice is taht the shifts for the benzene carbons all appear in a similar chemical shift range (115-130ppm)[11]. This is an expected trend for benzene based carbons in 13C NMR . However it must be noticed that the carbon in the ipso position provides a high downfield shift compared to the other bezene carbons of around 140ppm. This is due to the electron witdrawing nature of the tetrahedral substituents. To assign the isomers, the main chemical shift region to analyse and comapre to literature concerns the tetrahedral sp3 centre. The C-Br experimental shift had to be corrected by -12ppm due to spin orbital interactions and the correction is shown in the results table. Overall the experimental results for Isomer A show good agreement with the literature data with the largest difference between the two sets of data being around 5ppm (taking into consideration the C-Br correction). The main problems were encountered when considering Isomer B as this calculation had to be repeated several times due to anomalous results being obtained. Different molecular arrangements were tested and optimised using the above methodolgy, and the best results were obtained when the benzene ring was perpendicular to the C-Br bond with the O-H hydrogen tilted in towards the plane of the ring, as shown in the assignments above.
Problems and Considerations
If provided with these two 13C NMR, I believe they would be too similar to successfully separate Isomer A from Isomer B due to similar chemical shifts. It has been proved above that the computational methods used by Gaussian are not accurate enough to predict the NMR's even though this is a small molecule. Larger moelcules would encounter more difficulties due to many conformations available for the molecule to take. The experimental chemical shifts for the sp3 hybridized carbon atoms of the product are 28.2, 46.4, and 73.3 ppm[12]. The calculations from Gaussian can be used with a DEPT-135 NMR spectrum allowing the assigment of the structures. DEPT is "Distortionless Enhancement by Polarisation Transfer". DEPT-135 NMR spectrum allows one to distinguish between different orders of substitution on a carbon centre. In the DEPT-135 spectrum, signals for quaternary carbons are not observed due to the DEPT techninque which uses polarisation transfer whereby a signal is induced by the transfer of proton magnetisation onto the carbon. However it is possible to observe signals for carbons with an odd number of hydrogens. These are represented by positive peak and signals for methylenes (CH2) are represented by negative peak[13]. Therefore it is possible to distinguish between a C-Br carbon and a C-H carbon. Consider the DEPT-135 of Isomer A provided by B. Andersh et. al.[14] (shown below). As the carbon at the sp3 centre is a quaternary carbon, it is expected that the peak for this carbon will not be seen in the DEFT-135 spectrum and this is the case. The spectrum also shows a negative peak at 46.4ppm which shows that this carbon is part of a methylene group with a Br atom attached and a positive peak at around 28ppm shwoing the presence of the CH3 group. This DEPT spectrum allows us to show that Isomer A is the major product in the reaction.

Analyzing the Vibrational Spectrum
| IR Spectrum Isomer A | IR NMR Spectrum Isomer B | ||
|---|---|---|---|
 |
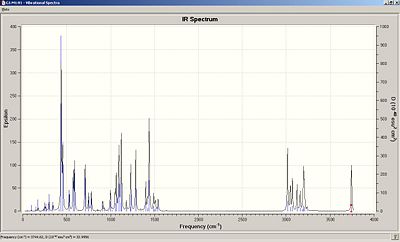 | ||
OH peak / cm-1 |
3735.60 |
3834.37
| |
Corrected / cm-1 |
3436.75 |
3527.95
|
Gaussian also allows the prediction of the vibrational spectra of Isomer A and Isomer B. Both isomers have characteristic peaks in their spectra which can be compared to literature data to show how effective molecular modelling can be in predicting vibrational spectra. The main peak which can be assessed is that of the -OH group in the isomers. Characteristically, depending on whether the alcohol shows a tendancy to hydrogen bond, the peak is observed in IR spectra at around 3500cm-1 and usually exhibits a very broad. This is the case in both product spectra with both -OH peaks appearing at around 3750cm-1. Errors in the predicted wavenumbers by Gaussian can be corrected by around 8% due to empirical errors. The resulting modification of the OH wavenumbers shows good correlation with the experimental data for the OH peak shown below.

References
- ↑ The mechanism of the Diels-Alder reaction, R. B. Woodward, T. J. Katz Tetrahedron , 1959 , 5 , 70 - 89
- ↑ K. Alder, G. Stein , Angew. Chem. , 1937 , 50 , 510
- ↑ Mechanistic aspect of Diels-Alder reactions : A Critical Survey. J. Sauer, R. Sustmann Angew. Chem., Int. Ed. Engl. , 1980 , 19 , 779 - 807
- ↑ Gill, G.; Pawar, D. M.; Noe, E. A, J. Org. Chem, 2005, 70, 10726-10731
- ↑ P. Camps et. al., Tetrahedron, 1997, 53, 9727-9734
- ↑ P. Camps et.al., Tetrahedron, 1997, 53, 9727
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319
- ↑ B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447
- ↑ S. R.K. Pingali et. al., Tetrahedron Letters, 2010, 51, 1383
- ↑ D. J. Porter et. al., J. Chem. Educ., 1995, 72, 1039
- ↑ Y. Kosugi et. al., Bulletin. Chem. Soc. Jap, 1978, 51, 2008
- ↑ B. Andersh et. al. J. Chem. Ed, 2008, 85, 102
- ↑ J. W. Akitt, NMR and chemistry: an introduction to modern NMR spectroscopy., 1992, 262
- ↑ B. Andersh et. al. J. Chem. Ed, 2008, 85, 102
- ↑ B. Andersh et. al. J. Chem. Ed, 2008, 85, 102