User:Aln09
Experiment 1C: Part 1
Conformational analysis using for Molecular Mechanics
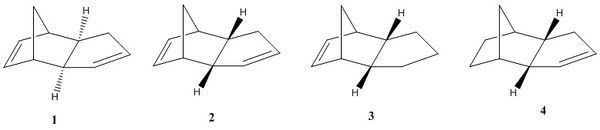
It has been experimentally shown that cyclopentadiene dimerises to form the endo product (2) preferentially over the exo product (1). The hydrogenation of the endo product results in either dimer 3 or 4. By use of Molecular mechanics, these dimers were modelled to determine whether the reaction was under kinetic or thermodynamic control, and to determine the most likely hydrogenation product. The dimers were optimised in ChemBio3D Ultra using the MM2 forcefield, table 1 shows the results.
| Dimer 1 | Dimer 2 | Dimer 3 | Dimer 4 | |
|---|---|---|---|---|
| Stretch (kcalmol -1) | 1.2851 | 1.2509 | 1.2348 | 1.0965 |
| Bend (kcalmol -1) | 20.5805 | 20.8477 | 18.9382 | 14.5243 |
| Stretch-Bend (kcalmol-1) | -0.8381 | -0.8358 | -1.9589 | -1.9589 |
| Torsion (kcalmol -1) | 7.6555 | 9.5109 | 12.1241 | 12.4974 |
| Non 1,4-VDW (kcalmol-1) | -1.4173 | -1.5439 | -0.8151 | -.8515 |
| 1,4 VDW (kcalmol -1) | 4.2334 | 4.3200 | 5.7290 | 4.5126 |
| Dipole(kcalmol -1) | 0.3775 | 0.4476 | 0.1631 | 0.1406 |
| Total Energy(kcalmol -1) | 31.8764 | 33.9975 | 35.9266 | 31.1520 |
Endo and Exo Formation
The calculations show that the exo dimer (1) has the lowest energy and is therefore thermodynamically more stable product, suggesting that if the reaction were under thermodynamic control it would be the major product. This is contrary to the experimental results, indicating that the reaction is under kinetic control, hence the transition state of the endo product must be lower in energy than that of the exo transition state. The plane to plane orientation of the two dimers in the endo transition state result in greater orbital symmetry between the LUMOs and HOMOs resulting in extra stabilistion of the transitions state. In the exo transition state there is no such favourable interaction resulting in a higher energy. The computed results are in agreement with the literature [1]. The results show that torsional strain is responsible for the difference in energy between the two dimers, with dimer 2 having 1.86kcalmol-1 more torsional strain than dimer 1.
Hyrdrogenation of Endo Product
The results show that dimer 4 is 4.77kcalmol-1 lower in energy than dimer 3, so is therefore the thermodynamically favoured product. Both double bonds in the endo dimer are equally substituted, so the selectivity does not arise from there, but it is a thermodynamic consequence. The significant difference in energy is due to the stretch-bend and bend energies, this can be explained by examination of the bond angles of the sp2 hybridised carbons. The C-C-C bond angles of the sp2 hybridised carbons in dimers 3 and 4 are 107.6o 113o respectively . This shows that the dimer 4 has a lower deviation from the idea sp2 bond angle of 120o, resulting in lower torsional strain.
Molecules 9 and 10 are atropisomers of the a key intermediate in the synthesis of the anti cancer drug Taxol. The isolation of atropisomers is the result of a high restriction to rotation about a single bond. The resulting atropisomers differ in position of the carbonyl group. The nature of the 6 membered carbon rings means that each isomer can exist as four different conformers: two twist boats and two chairs. All four confomers for each isomer were modelled and the total energies are displayed in Table 2:

| Intermediate 9 | Intermediate 10 | |
|---|---|---|
| Chair A (kcalmol -1) | 51.3067 | 52.6180 |
| Chair B (kcalmol -1) | 47.8395 | 42.6828 |
| Twist Boat A (kcalmol-1) | 53.0099 | 46.4335 |
| Twist Boat B (kcalmol -1) | 53.0814 | 48.4057 |
As expected, for both isomers the chair conformation was the lowest energy and most thermodynamically stable. The results show the chair conformations of Intermediate 10 is lower in energy than Intermediate 9, a more detailed breakdown in the origins of this energy difference is shown in Table 3:
| Isomer 9 | Isomer 10 | |
|---|---|---|
| Stretch (kcalmol -1) | 2.7848 | 2.6205 |
| Bend (kcalmol -1) | 16.5411 | 11.3390 |
| Stretch-Bend (kcalmol-1) | 0.4304 | 0.3432 |
| Torsion (kcalmol -1) | 18.2515 | 19.6722 |
| Non 1,4-VDW (kcalmol-1) | -1.5525 | -2.1620 |
| 1,4 VDW (kcalmol -1) | 13.1092 | 12.8721 |
| Dipole(kcalmol -1) | -1.7248 | 12.8721 |
The bend energy is responsible for the difference in energy between the two chair conformations of 9 and 10, with 10 having a bend energy 5kcalmol-1 less than 9 (all the other parameters are roughly equal). In intermediate 10, the bond angle of the sp2 hybridised carbon (i.e. the O-C-C bond) is ~ 120o, the optimum angle. However the same bond angle in intermediate 9 is ~115o which results in steric hindrance. Hence through calculation and direct analysis of the structures, it is clear that intermediate 10 is the most thermodynamically stable isomer.
Molecules 9 and 10 are hyperstable alkenes, and react at a much slower rate than their parent alkenes. The hyperstability arises from the position of the double bond at the bridge head, making the C=C unreactive[2].
Spectroscopic Simulation of Taxol Intermediates using Quantum Mechanics
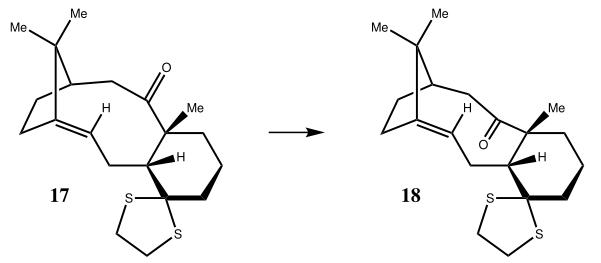
Molecules 17 and 18 are also derivatives of taxol. Molecule 18, the derivative of the more stable molecule 10, was modelled and using quantum mechanics the 1H and 13C spectra were calculated. Again due to nature of the cyclohexane ring, different conformations of the molecule can exist. The lowest energy chair (64.3137kcalmol-1) and lowest energy boat (64.1657kcalmol-1) conformations were modelled and optimised under an MM2 forcefield in ChemBio3D Ultra and their spectra was calculated. Note, the spectra were modelled in a Benzene solvent in order to make a more accurate comparison with the literature.
| Literature[3] (ppm) | Boat Conformer (ppm) | Chair Conformer (ppm) |
|---|---|---|
| 5.21 (m,1H) | 5.27 (1H) | 5.95 (1H) |
| 3.00-2.70 (m, 6H) | 3.29 (1H) | 3.17-3.18 (2H) |
| 2.70-2.35 (m, 4H) | 3.19 (1H) | 2.90 (1H) |
| 2.20-1.70 (m, 6H) | 3.06 (1H) | 2.80-2.81 (3H) |
| 1.58 (t, 1H) | 2.33-2.86 (2H) | 2.67 (1H) |
| 1.50-1.20 (m, 3H) | 2.45 (1H) | 2.58(1H) |
| 1.10 (s, 3H) | 2.35 (1H) | 2.50 (1H) |
| 1.07 (s, 3H) | 2.27 (2H) | 2.40 (1H) |
| 1.03 (s, 3H) | 2.14 (1H) | 2.30-2.33 (2H) |
| 1.92-1.97 (2H) | 1.97-2.00 (3H) | |
| 1.82 (2H) | 1.82 (1H) | |
| 1.69 (2H) | 1.52-1.62 (4H) | |
| 1.66 (2H) | 1.27 (4H) | |
| 1.58 (1H) | 1.20-1.23 (2H) | |
| 1.53 (1H) | 0.97 (2H) | |
| 1.47 (1H) | 0.91 (1H) | |
| 1.44 (1H) | 0.61 (1H) | |
| 1.34 (1H) | - | |
| 1.16 (1H) | - | |
| 0.94-1.06 (5H) | - |
By comparison of the 1H spectra for the boat and chair confirmation, it can be seen that both spectra share a similar pattern of peaks, however the chemical shifts do not strongly agree with differences of up to 0.7ppm. This is as expected, due to the different chemical environments experienced by the hydrogens in the different conformations.
The data shows that the boat conformation most closely matches the literature, as all calculated signals lie within 0.4ppm of the literature. The computed spectra suggests there are many more chemically distinct environments which appear as multiplets in the literature, due to very close and overlapping chemical shifts making the multiplicity too difficult to determine accurately. In contrast, the chair conformation loosely matches the literature, with values up to 0.7ppm away from the literature. This suggests the conformation recorded in the literature is the boat conformation, which would agree with the molecular modelling which indicates the boat conformation is infact the lowest in energy and hence most thermodynamically stable.
References
- ↑ W. L. Jorgensen, D. Lim, J. F. Blake,J. Am. Chem. Soc., 1993, 115 (7), 2936-2942.Template:DOI:10.1021/ja00060a048
- ↑ A. B. McEwen, P. R. Schleyer,J. Am. Chem. Soc., 1986, 108, 3951-3960.
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
Experiment 1C: Part 2 - Analysis of the Properties of Synthesised Alkene Epoxides
Crystal Structures of Shi and Jacobsen Pre-Catalysts
Shi catalyst selectively catalyses the trans epoxidation of trans alkenes, where as Jacobsen catalyst selectively catalyses the cis epoxidation of the cis alkenes. Using Conquest the crystal structures of Shi and Jacobsen pre-catalysts were obtained from the Cambridge Crystal Database and the structures anaylsed to explain their selectivity.
Shi Pre-Catalyst
Shiprecusor21 |
It can be seen from the crystal structure of the pre catalyst (hence the catalyst itself) the dioxirane group is neighboured by two bulky 5 membered rings, one pointing up and the pointing down relative to the cyclohexane ring. Hence only trans alkenes can approach the dioxirane group without experiencing significant steric hindrance; cis alkenes would experience significant steric hindrance from at least one of the 5 membered rings. This is a clear indication of the trans selectivity of the shi catalyst.
The pre-catalyst has two anomeric centres; for the anomeric centre located on the 5 membered ring the C-O bond lengths are not equal, one is 0.141nm and the other 0.144nm. The oxygens in the 5 membered ring are in an antiperiplanar arrangement, hence hyperconjugation of the oxygen lone pair occurs with the C-O σ* bond. Occupation of the C-O antibonding orbital results in a weakening on the C-O bond hence the bond length increases. For the other anomeric centre located on the 6 membered ring, both C-O bonds are 0.141nm in length. The oxygen of the six membered ring is forced into an equatorial position (a phenomena known as the anomeric effect), as a result there is a lack of anti periplanar arrangement and delocalisation of the oxygen lone pair by hyperconjugation is not possible, and the bond lengths remain equal.
Jacobsen Pre-Catalyst
Jacobsen23 |
In Jacobsen catalyst the calculated distance between the hydrogens of two adjacent tertiary butyl groups is 0.242nm, this is in the region for attractive Van der Waals interactions hence stabilises the molecule. These stablising interactions in combination with the other tertiary butyl groups bound to the phenyl ring effectively block the side on approach of the alkene to the metal-oxygen bond. Hence the alkene must approach over the cyclohexane moiety resulting in cis selectivity for epoxidation, and cis selectivity for the alkene substrates to reduce steric clashes.
Calculated NMR Spectra of Epoxides
Styrene and trans-stilbene were the alkenes chosen to undergo epoxidation. The resulting epoxides; (R)-styrene oxide, (S)-styrene oxide, (R,R)-trans-stilbene oxide and (S,S)-trans-stilbene oxide were drawn and optimised under a MMR94s forcefield in Avagadro. Then using Quantum mechanical methods the 1H (400MHz) and 13C (100MHz) for all epoxides (in chloroform solvent) were computed, the results are shown in the tables below.
Styrene Oxide
| Literature[1] 1H NMR (ppm) | Calculated 1H NMR (ppm) | Literature[1] 13C NMR (ppm) | Calculated 13C NMR (ppm) | |
|---|---|---|---|---|
| 7.24-7.33 (m, 5H) | 7.45-7.51 (4H) | 137.5 | 135.13 | |
| 3.80 (dd, 1H, J=2.8, 4.1Hz) | 7.30 (1H) | 128.2 (2C) | 124.14 | |
| 3.08 (dd, 1H, J=4.1, 5.6Hz) | 3.66 (1H) | 128.0 | 123.42 | |
| 2.75 (dd, 1H, J=2.8, 5.6Hz) | 3.12 (1H) | 125.3 (2C) | 122.96 (2C) | |
| - | 2.54 (1H) | 51.1 | 118.27 | |
| - | - | 51.0 | 54.06 | |
| - | - | - | 53.48 |
The computed 1H and 13C NMR are in good agreement with the literature; the computed chemical shifts for 1H are within ~0.2ppm and 13C are within ~3ppm which is a good indicator that the correct structure has been modelled. In the literature the 5H multiplet at 7.24-7.33pm corresponds to the aromatic protons on the benzene ring, however Quantum mechanics has predicted these protons to appear as a two distinct environments: 4H at 7.45-7.51ppm and 1H at 7.3ppm. The 7.3ppm corresponds to the para proton on the benzene ring, which does not experience a strong deshiedling effect from the electron withdrawing oxygen in the epoxide group.
In the 13C, the doubly degenerate peak at 128.2ppm is equivalent to the calculated peaks at 124.14 and 123.42ppm. This is suggests that these carbons in reality are not chemically distinct. Both the 13C and 1H spectra for the two enantiomers was identical, hence is shows that enantiomers do not produced different spectra.
Trans-Stilbene Oxide
| Lierature[1] 1H NMR (ppm) | Calculated[2] 1H NMR (ppm) | Literature[1] 13C NMR (ppm) | Calculated[2]13C NMR (ppm) | |
|---|---|---|---|---|
| 7.25-7.59 (m, 10H) | 7.569 (2H) | 137.1 (2C) | 134.088 (2C) | |
| 3.87 (s, 2H) | 7.505 (8H) | 128.5 (4C) | 124.22 (2C) | |
| - | 7.488 (8H) | 128.3 (2C) | 123.516 (2C) | |
| - | 7.467 (8H) | 125.5 (4C) | 123.212 (2C) | |
| - | 7.44897 (8H) | 62.8 (2C) | 123.078 (2C) | |
| - | 3.535(2H) | - | 118.263 (2C) | |
| - | - | - | 66.478 (2C) |
The computed chemical shifts for the 1H spectra are within ~ 0.2ppm of the literature, hence is can be stated with confidence that calculations and literature are in good agreement. The protons responsible for the calculated peaks ranging from 7.25-7.496 represent the 10 aromatic protons on the two benzene rings. The calculation has predicted 4 distinct chemical environments for these protons, which could be due to the deshielding effects of the electron withdrawing oxygen and limited rotation. However the literature shows that these appear as a multiplet, hence the environments are not distinct.
The computed chemical shifts for the 13C spectra are within a range of 3-5ppm of the literature values. There is one more literature value, suggesting that there is one more chemically distinct carbon, due to limited rotation which has not been accounted for by quantum mechanics. Overall the the calculated and literature values for both spectra are in good agreement, so it can be said with certainty that the correct structure has been modelled.
Both the 13C and 1H spectra for the two enantiomers was identical, hence it is clear that absolute configuration does not have a significant impact on the NMR spectra.
Overall, the literature data was in good agreement with the computational values for the NMR spectra for all four modelled molecules. It can be concluded that the absolute confirguration does not have a significant impact (if any) on the NMR spectra recorded, hence NMR is not a viable technique for assigning which enantiomer has been produced, and that other methods such as optical rotation must be used to assign the absolute configuration.
Assigning the Absolute Configuration of Epoxide Products
Theoretically two enantiomers for each reaction can be formed, so it is important to be able to correctly assign which enantiomer has been formed. As shown in the previous section, NMR is not a viable tool to achieve this, hence analysis of the optical rotations allows assignments to be made with a high degree of certainty. To achieve this, the two possible enantiomers of styrene oxide and tran-stilbene oxide were drawn out and optimised in ChemBio3D Ultra to determine the stereochemistry.
| R-styrene[3] | S-styrene[4] | R,R-trans-stilbene[5] | S,S-trans-stilbene[6] | ||
|---|---|---|---|---|---|
| 589nm | -70.93o | 71.02o | 300.92o | -300.72o | |
| 365nm | -250.66o | -251.00o | 1254.78o | -1253.96o |
Literature values for the optical rotation of the enantiomers was inconclusive, as both positive and negative ORPs have been reported for the same enantiomer. Hence it has not been possible to assign the absolute configuration of the epoxides.
Using the Calculated Properties of Transition State for the Reaction cis/trans-β-methylstyrene
The transition states of the epoxidation of trans-β-methylstyrene with the Shi catalyst and of cis-beta methyl styrene with the Jacobsen catalyst were modelled to find the Gibbs free energies. For both reactions, difference in Gibbs free energy between the two possible enantiomeric products was determined, allowing calculation of the ratio of enantiomers (the equilibrium constant) and enantiomeric excess. As there were more than one transition state for each epoxidation reaction, calculations were performed using the lowest energy transition states as this is the most likely pathway the reaction would proceed. Table X shows the results.
| ΔΔG for R,R to S,S Interconversion (trans-β-methyl styrene Oxide Transition States) | Equilibrium Constant K for R,R to S,S Interconversion (trans-β-methyl styrene Oxide Transition States) | ΔΔG for S,R to R,S Interconversion (cis-β-methyl styrene Oxide Transition States) |
Equilibrium Constant K for S,R to R,S Interconversion (cis-β-methyl styrene Oxide Transition States)!! |
|---|---|---|---|
| -20.218977kJmol-1 | 3486.652116 | -22.3141262kJmol-1 | 8118.640311 |
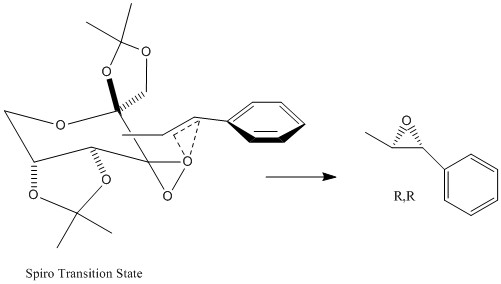
The Shi catalyst utilises the mechanism of dioxirane epoxidation, achieved by the transfer of an on oxygen from the diroaxane group attached to the cyclohexane group in an equatorial position, the most thermodynamically stable conformation[7]. This results in enantioselective epoxidations of trans-disubstituted alkenes, in this case trans-β-methylstyrene. In this reaction two types of transition states can be formed: spiro and planar, (which refer to the orientation of the trans alkene) and result in opposite configurations, R,R and S,S respectively [8]. Hence factors affecting the stabilisation of these transition states directly impact the enantiomer produced.
The results show the ratio of R,R/S,S enantiomers is very large (3486.652116), giving an enantiomeric excess of 99.97% in favour of R,R-trans-β-methylstyrene oxide. The calculations are agreement with the literature [8] [9], where Shi et al reported enantiomeric excess values upwards of 82% ee for the R,R enantiomer in a range of different solvent systems. However a direct comparison cannot be made as our solvent system was water, which was not included in the solvents in the literature. From this result it can be concluded spiro transition state is lower in energy and hence more thermodynamically stable than the planar transition state.
This increased stablisation can be explained by analysis of electronic and steric interactions. In the spiro transition state there is favourable orbital overlap between the non bonding oxygen lone pair and the π* orbital of the C=C bond. The conjugated phenyl group of trans-β-methyl styrene lowers the energy of the π* orbital, decreasing the HOMO-LUMO gap and promoting stability of the transition state. The figures below depict the lowest energy transition spiro and planar transition states[8]. It is clear that spiro transition state is favoured sterically, as the phenyl group of trans-β-methyl styrene is in the endo position with respect to the Shi catalyst, hence steric clashes are minimal (the phenyl group is planar so there is minimal steric clashes between it and the ketal group[9]). Conversley the planar transition state is actively disfavoured, as it can be seen in the figure below there are significant steric clashes between the exo phenyl group and cyclohexane ring of the shi catalyst.
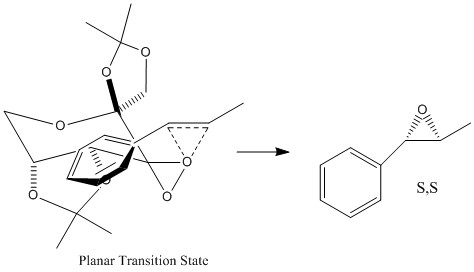
For the epoxidation of the cis-β-methyl styrene with Jacobson's catalyst equilibrium constant for the interconversion of the S,R to R,S was also very high, with an enantiomeric excess of 99.99%. The literature also reports an enantoimeric excess of 92%, confirming that S,R-cis-β-methylstyrene oxide is the thermodynamically favoured enantiomer.
Investigating the Non-Covalent Interactions in the Active Site of the Reaction Transition State
Non Covalent Interactions (NCIs) including hydrogen bonds, electrstatic interactions and Van Der Waals forces between a catalyst and its substrate are key to understanding the stability of the transition state, and can provide an indication to the origins of its selectivity. The NCIs of the 4th R,R (endo) transition state for the epoxidation of trans-β-methylstyrene catalysed by the Shi catalyst were modelled and the resulting structure is shown below.
Orbital |
A key interaction to note is the electrostatic potential isosurface between the oxygens in the 5 membered ring is red, indicating strong repulsive forces. This is due to the strong repulsion of the oxygen lone pair electrons that are forced into close proximity due to the nature of the ring. There are favourable attractive interactions between the hydrogens on the 5 membered ring and the hydrogen on the alkyl chain of the alkene substrate. As the interactions are between hydrogen atoms, they are likely to be Van Der Waals forces, and although individually they are weak, collectively they are strong and overcome the repulsion between the oxygen lone pairs to stabilise the transition state. Finally the ring between the alkene substrate and the catalyst represent bond formation in the transition state and is highly complex.
Investigation of the Electron Topology (QTAIM) in the Active Site of the Reaction Transition State
The 2nd S,S transition state of the expoxidation of the trans-β-methylstyrene by the Shi catalysed underwent QTAIM anaylsis to investigate the electron distribution in covalent, and more interesting non covalent interactions.
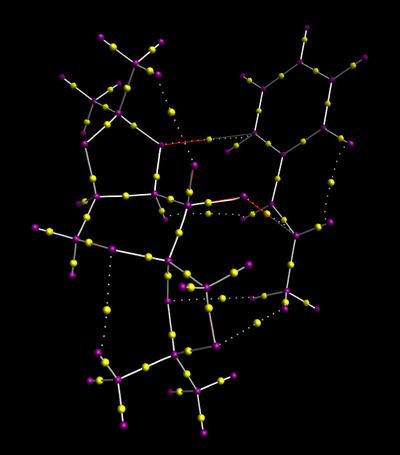
Bond Critical Points (BCPs) represent the region where electron density is most concentrated. On the image above, it can be seen for homonuclear covalent bonds the peak in electron density is equidistant between the nuclei. However for the heteronuclear bonds the region of highest electron density is observed to be closest to the most electron withdrawing nucleus. This is most markedly observed in the C-O bond, where the highest electron density is observed very close to the oxygen atom due to its high electronegativity. The most interesting BCP is on the non covalent interaction between the oxygen in the dioxirane group of the Shi catalyst and the alkene group of the substrate, shown by arrow 1. This indicates new bond formation in the transition state.
New Candidates
References
- ↑ 1.0 1.1 1.2 1.3 C. Wiles, M. J. Hammond, P. Watts,Beilstein Journal of Organic Chemistry., 2009, 5, No 27.Template:DOI:10.3762/bjoc.5.27
- ↑ 2.0 2.1 A. Nofer, "R- Styrene H NMR", DSpace, 2013,DOI:10042/25852
- ↑ A. Nofer, "R- Styrene ORP", DSpace, 2013,DOI:10042/25848
- ↑ A. Nofer, "S- Styrene ORP", DSpace, 2013,DOI:10042/25849
- ↑ A. Nofer, "RR-trans-stilbene ORP", DSpace, 2013,DOI:10042/25851
- ↑ A. Nofer, "SS-trans-stilbene ORP", DSpace, 2013,DOI:10042/25850
- ↑ a. Burk, P. Dillon, K. Martin and T.W. Hanks,Journal of Chemical Education, 2000, '77, 271-272.Template:DOI:10.1021/ed077p271
- ↑ 8.0 8.1 8.2 Y. Shi,Acc. Chem. Res., 2004, 37, 488-496.Template:DOI:10.1021/ar030063x
- ↑ 9.0 9.1 Z. Wang, Y. Tu, M. Frohn, J. Zhang and Y. Shi,J. Am. Chem. Soc., 1997, 119, 11224-11235.Template:DOI:10.1021/ja972272g
