Talk:Mod:Hunt Research Group/mman
1. System: MMAN
Formula: [CH3NH3][NO3]
No. of atoms: 576
No. of ion pairs: 48
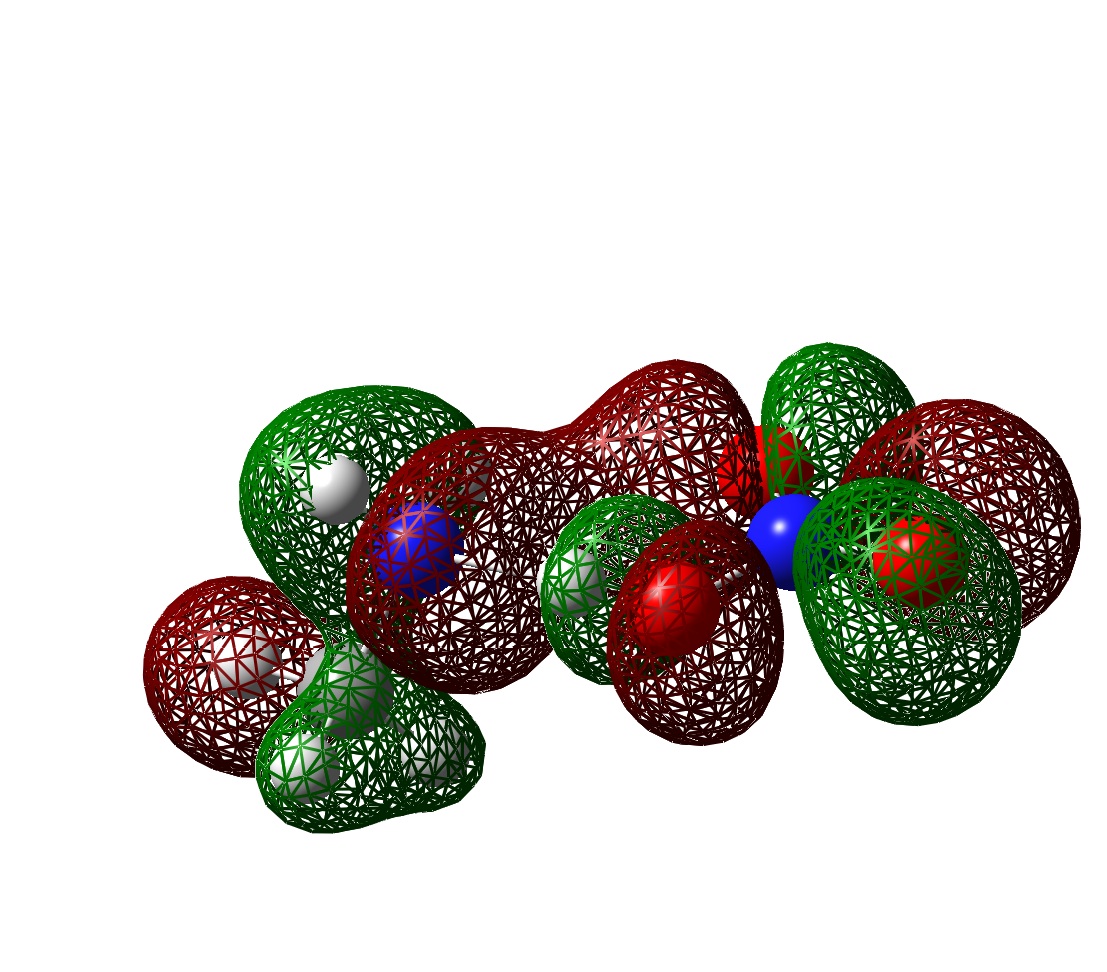
2. Gaussian HOMO
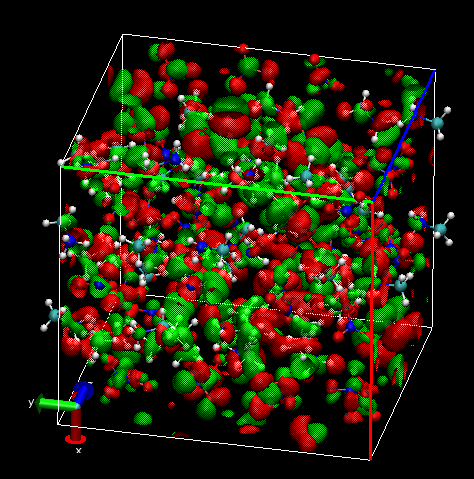
3. CPMD HOMO
4. MO comparison between that of one ion pair using Gaussian and PBC using CPMD
The shape of the orbital inside each molecular is similar in the isolated pair and in the condensed system, although the HOMO in the liquid system is clearly extended. Can see if the interaction between individual molecules is bonding, anti-bonding or non-bonding. There is certain degree of localisation in the condensed system because of disorder. It looks quite localised using sufficiently large isosurface parameters. Also, there seems to be large voids between molecular units on which the orbital is localised.
To do: quantify the interactions between the molecular units in the condensed system: strong or weak? beta values? (can actually separate the system into individual mmans, and then compute EMOs for each of them.)
5. Wannier analysis (done)
6. Use of EMO method:
The HOMO is not localised in an absolute sense, i.e. it still is an extended state in the condensed sample (i.e. sits on more than one molecular unit). So the EMO method is the only way to get information on the electronic structure of each individual molecular unit.
7. Development of EMO method: