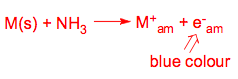StudentWiki:Periodicity
Periodicity
The contents of this course are taken directly from Dr. Rob Davies' course (2010 - 2011) and are copyright of Imperial College London.
Introduction

The course will be looking over the properties, bonding and structures of the s and p block elements and their compounds. Each Group within the s and p block of the periodic table will be considered in turn, with particular attention being placed upon the trends down the Group. In addition we will note the changes that occur along the Rows and the patterns of chemical behaviour that develop. Knowledge of concepts covered in previous lecture courses including types and shapes of orbitals, electronegativity, hybridisation and VSEPR theory is required for this course. The s-block elements are defined as those in vertical Groups 1 and 2 for which the valence electrons are only in s orbitals, whereas the p-block consists of elements with valence electrons in both s and p orbitals (Groups 13-18). The valence electrons for the transition elements are in s, d and f orbitals and have been considered separately.
General Principles
Ionic Bonds
An ionic compound consists of positive and negative ions with no sharing of electrons (e.g. Na+Cl-). A large electronegativity difference (>1.7) indicates charge separation and principally ionic bonding. This value of 1.7 is based on empirical calculations carried out by Pauling. Ionic lattices are bonded almost exclusively by electrostatic means. The strengths of these interactions depend on the charges and sizes of the ions. The greatest interactions occur when the cation and anion are of similar sizes and are highly charged.
| Sodium chloride |
|---|
| Electronegativity of Na: χ(Na) = 0.9 Electronegativity of Cl: χ(lL) = 3.0 |
| Difference in electronegativity = 2.1 |
| IONIC |
Covalent Bonds
A covalent bond is a bond between atoms involving sharing of electrons (e.g., N2, H-Br).
A low electronegativity difference (<1.7) indicates covalent or polar covalent bonds.
| Nitrogen, N2 | Hydrogen bromide, HBr |
|---|---|
| Electronegativity of N: χ(N) = 3.0 |
Electronegativity of H: χ(H) = 2.1 Electronegativity of Br: χ(Br) = 2.8 |
| Difference in electronegativity = 0 | Difference in electronegativity = 0.7 |
| 100% COVALENT | 88% COVALENT |
Electronegativity also indicates what the ionic contribution to the bond energy is. Where there is an ionic contribution to the bond there is a strengthening of the bond and a reduction in bond length below what would be expected purely on covalent interactions. The covalent radius of H = 0.37 Å and of Br = 1.14 Å. Based on purely covalent interactions we would expect a H-Br bond length of 1.14 + 0.37 = 1.51 Å. However, the experimentally observed value = 1.41 Å, which is shorter and stronger due to an ionic contribution.
The greatest overlap between orbitals occurs where orbitals are well matched in terms of size and energy.
Hence σ and π bond energies decrease going down a group.
For example, for Group 14 single bond lengths, the bond strengths change as follows:
| Bond | Bond Energy |
|---|---|
| C-C | 348 kJmol-1 |
| Si-Si | 226 kJmol-1 |
| Ge-Ge | 188 kJmol-1 |
| Sn-Sn | 151 kJmol-1 |
| Pb-Pb | 89 kJmol-1 |
Hybridisation and VSEPR Theory
You should already be familiar with the concept of hybridisation theory and the use of VSEPR theory for predicting the shapes of molecules.
Each set of hybrid orbitals is associated with a particular shape, although this may not coincide with the molecular shape if lone pairs also have to be accommodated.
Some of the most common forms of hybridisation and their shapes are:
| Hybridisation | Shape |
|---|---|
| sp | Linear |
| sp2 | Trigonal Planar |
| sp3 | Tetrahedral |
| sp3d | Trigonal Bipyramidal |
| sp3d2 | Octahedral |
Therefore we can predict the hybridisation scheme for a particular atom by looking at its pseudostructure (i.e., its shape including lone pairs).
This psuedostructure can either be found using spectroscopic methods or predicted with the use of VSEPR theory.
Hybridisation of sulphur tetrafluoride:

Oxidation States
"The apparent (or formal) number of electrons added to or removed from an atom when it forms a compound."
This is a formal assignment and may not reflect the real nature of electron use in bonds (the valence).The oxidation state of an element is taken to be zero, irrespective of whether the element exists as atoms (e.g. Ne), molecules (e.g., O2, P4) or an infinite lattice (e.g., Si). In addition, in the assignment of oxidation states to elements in a compound, any homonuclear bond is ignored. High positive oxidation states are unlikely to be ionic.
Group 1 - Alkali Metals
The Elements
| Alkali Metal | Symbol | Electron Configuration | Melting Point |
|---|---|---|---|
| Lithium | Li | [He]2s1 | 181 °C |
| Sodium | Na | [Ne]3s1 | 98 °C |
| Potassium | K | [Ar]4s1 | 64 °C |
| Rubidium | Rb | [Kr]5s1 | 39 °C |
| Caesium | Cs | [Xe]6s1 | 29 °C |
All the alkali metals are one-electron metals. The atoms pack together as an infinite lattice and the metallic bonding can be considered as a matrix of cations in a sea of electrons (this model of bonding is poor for most metals but suffices here). The metals become softer and have lower melting and boiling points down the group. This is because the strength of metal-metal bonding is dependent upon valence s-orbital overlap. Increasing s-orbital size down the group results in their being more diffuse, leading to a poorer overlap and hence weaker bonds.
Alkali metals dissolve in liquid ammonia to give blue solutions that are paramagnetic (i.e. they contain unpaired electrons).
Each solution of metal in liquid NH3 occupies a volume greater than the sum of the volumes of the metal plus solvent. These data suggest that electrons occupy cavities of radius 3-4 Å. These solutions are strongly reducing and have found applications as reducing agents.
All of the Group 1 metals react with water. Lithium reacts slowly, sodium vigorously, potassium causes the hydrogen to ignite, and rubidium and caesium react explosively.
You can watch their respective reactions with the following YouTube video: http://www.youtube.com/watch?v=cqeVEFFzz7E (look at dat sexy Hammond with dem sexy elements :P). Their reaction with water is given by the following general equation:
The Compounds
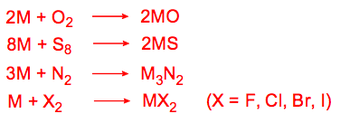
All the metals react with the halogens and hydrogen when heated:
The highly electropositive nature of the Group 1 metals results in their compounds being predominately ionic in nature [e.g., K+I-, (Na+)2S2- and (Li+)3N3-]. Therefore most Group 1 metal compounds exhibit properties symptomatic of ionic compounds, i.e. they are crystalline, hard, have high melting points, and dissolve in polar solvents such as water to give conducting solutions.
It has proved possible to wrap up M+ ions in stable complexes using polydentate crown ether ligands. Such ligands can impart solubility to complexed ions in non-polar solvents. For example, the powerful oxidant KMnO4 can be made soluble in toluene, which can be of use in organic synthesis. Nature also uses biological analogues to these ligands to enable Na+ or K+ ions to be stored or transported across ‘non-polar’ membranes. This is due to the metal cation being surrounded by an “organic sheath” rendering it soluble in organic non-polar media.
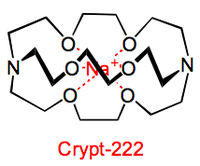
Three dimensional polydentate ligands called cryptands are also known.
These ligands are capable of completely encapsulating the metal cations, hence protecting the complexed metal cation even more effectively than crown ethers do.
These cryptands are also able to promote the disproportion reaction of all the Group 1 metals (except Li) to their +1 and –1 oxidation states.
The applications of the alkali metals and their compounds are manifold ranging from the use of sodium chloride (NaCl) in food products and winter road clearance to the use of lithium carbonate (Li2CO3) in the treatment of manic depression (note however that large amounts of lithium salts can damage the central nervous system!). Lithium is also increasingly used in solid-state batteries, due to its high negative reduction potential and its small size and hence mobility.
Group 2 - The Alkaline Earth Metals
The Elements
| Alkali Earth Metal | Symbol | Electron Configuration |
|---|---|---|
| Beryllium | Be | [He]2s2 |
| Magnesium | Mg | [Ne]3s2 |
| Calcium | Ca | [Ar]4s2 |
| Strontium | Sr | [Kr]5s2 |
| Barium | Ba | [Xe]6s2 |
The Group 2 elements are all two-electron metals. Like the Group 1 metals their hardness and boiling points decrease down the group although the different metallic structures of the elements results in this decrease not being regular. The melting points are higher for Group 2 elements than Group 1 elements since there are now two electrons participating in the metallic bonding. Again, similar to Group 1 metals the reactivity increases down the group due to the weaker nature of the M-M bonds, decreasing ionisation energies and decreasing lattice enthalpies.
Like the Group 1 metals they dissolve in ammonia to give blue solutions containing solvated electrons.
Beryllium and Magnesium are passivated and kinetically inert to O2 and H2O at ambient temperatures.
However, the other members of the group react with water to liberate H2, and Mg can react with steam or hot water:
When heated, all the Group 2 metals combine with O2, N2, sulphur or halogens.
The Compounds
The Group 2 species exist exclusively in the +2 oxidation state. Mg, Ca, Sr and Ba all form predominately ionic compounds (like the Group 1 metals), whereas Be tends to form covalent compounds. For example, molten BeF2 is practically a non-conductor of electricity. This is because Be2+ has a very high charge density and therefore polarises all anions and ligands resulting in the formation of polar-covalent compounds.
In the vapour state BeCl2 is monomeric and has a linear structure. It is sp hybridised and is electron deficient since it only contains 4 electrons in its valence shell.
In the solid state it forms infinite chains with tetrahedral Be centres and longer Be-Cl distances than in the monomer. In this form, the Be is sp3 hybridised. Each chlorine atom donates a pair of electrons into an empty sp3 orbital on an adjacent Be atom, thereby helping relieve the electron deficiency.
In aqueous solutions:
- Be2+ is always 4 coordinate
- Mg2+ is either 4 or more usually 6 coordinate
- Ca2+ is either 6 or 8 coordinate.
Two important ligands are [EDTA]4- and [P3O10]5- which both form very stable complexes with Mg2+ and Ca2+ ions and are used in water-softening to remove these ions.
Group 3 - The Boron Group
The Elements
| Element | Symbol | Electron Configuration |
|---|---|---|
| Boron | B | [He]2s22p1 |
| Aluminium | Al | [Ne]3s23p1 |
| Gallium | Ga | [Ar]3d104s24p1 |
| Indium | In | [Kr]4d105s25p1 |
| Thallium | Tl | [Xe]4f145d106s26p1 |
Boron has a very stable, inert, macromolecular structure in which the bonding is somewhere between metallic (free electrons) and covalent (localised electrons) and therefore it acts as a semi- conductor. All the other elements in the group are metallic. The lack of a metallic structure for boron reflects in reluctance to form positive ions due to its high ionisation energies.
There are at least five distinct allotropes of Boron known, all of which contain B12 cluster units that in most cases are accompanied by other boron atoms lying outside the icosahedral cages. An allotrope (from the Greek "other" + "grow") is the term given to the various forms of existence of the same compound.
Aluminium is resistant to corrosion since it readily forms a very adherent and impermeable oxide layer.
Unlike Groups 1 and 2 the atom radii of the elements do not increase uniformly down the group, since Al and Ga are similar in size, as are In and Tl.
This is due to the d-block (Al→Ga) and f-block (In→Tl) contractions. These contractions occur due to the presence of a complete d or f shell of orbitals that shield poorly the greatly increasing nuclear charge. The valence electrons therefore feel a greater pull towards the nucleus, thus contracting the size of the atom.
The Compounds
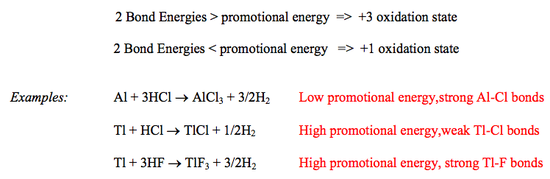
Virtually all stable compounds of B and Al contain the elements in the oxidation state +3 (corresponding to the use of all the valence electrons) but a few compounds of Ga and In and many compounds of Tl contain the elements in oxidation state +1. This is due to the "Inert Pair Effect”.
Down the group the separation between s and p orbitals increases due to s-electrons being able to penetrate closer to the nucleus because of feeling a greater effect of the increasing nuclear charge. Hence, the promotional energy from ns2np1 to ns1np1np1 increases down the group. Also bond energies decrease down the group as the atomic sizes increase and more diffuse orbitals are used for bonding. Therefore at the bottom of the group the energy required for promotion is greater than the energy that would be released by the formation of two new bonds.
Boron trihalides (BX3) can generally be prepared by halogenation of boron oxides or by direct reactions with halogens.
All BX3 species are monomeric and have trigonal planar structures (sp2 hybridised boron).
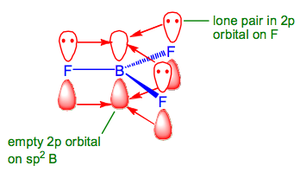
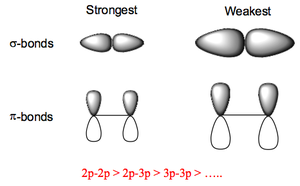
All the B-X bonds are shorter and stronger than expected. For example for B-F we expect a bond length of 1.61 Å (sum of covalent radii of B and F), or taking into account the ionicity of the bond (due to the electronegativity difference between B and F) 1.50 Å. However, the real observed bond length is 1.31 Å. This can be explained by looking at the bonding present in BX3 species:
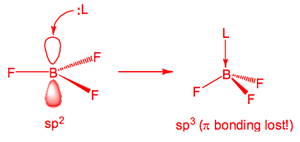
This is due to π-donation from the F to the B due to good p-orbital overlap.br /> Despite this extra stabilisation all BX3 are still electron deficient and will accept two electrons into the vacant p orbital so as to form an 8e octet, i.e., they are Lewis Acids. The order of Lewis acidity is BI3 > BBr3 > BCl3 > BF3.
For BI3 the 2p(B)-6p(I) π-overlap is much weaker due to the size/energy mismatch, and is therefore more easily broken.
BI3, BBr3 and BCl3 are all hydrolysed in water to give boric acid:
In the case of BF3, because of the strength of the B-F bonds the hydrolysis reaction stops at the adduct:
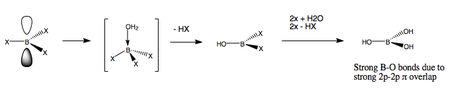
Unlike the trihalides, BH3 is not monomeric but exists as dimers B2H6 (diborane).
Its structure cannot be accounted for properly by Valence Bond theory.
However MO theory provides an explanation (this model for diborane was first developed by Longuet-Higgins whilst still and undergraduate!).
In diborane each boron is sp3 hybridised and forms normal 2-electron 2-centre (2e-2c) bonds with the terminal H atoms as well as 2-electron 3-centre (2e-3c) B-H-B bridging bonds.
B2H6 is highly reactive (explosive) with O2 and H2O forming boron oxides (B2O3) or hydroxides [B(OH)3]. The highly stable B-O bonds are the thermodynamic driving force behind these reactions. MX3 species of the other Group 3 elements are all Lewis acids and the Lewis acid strengths decrease down the group in the order Al > Ga > In. Al3+, Ga3+ and In3+ ions all have a high charge density and can polarize anions so AlBr3, for example, is covalent although AlF3 is more ionic.
Note that dimerisation to relieve the electron deficiency is preferred to the formation of π-bonds as seen in BCl3 since the 3p-3p π overlap in AlCl3 is not nearly as good (i.e. strong) as the 2p-3p π overlap in BCl3. AlCl3 is a volatile solid giving Al2Cl6 molecules in the gas phase: