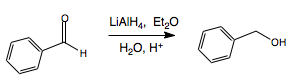StudentWiki:Carbonyls-Carboxyls
Carbonyls and Carboxyls
Physical Properties of Carbonyls and Carboxyls
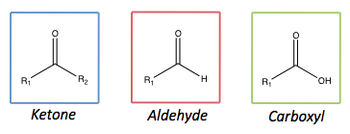
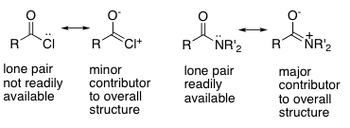
A carbonyl compound or carboxyl group contain a C=O double bond. This is a region of fairly high electron density that is polarised towards the oxygen atom due to its higher electronegativity. The electronegativity of oxygen is 3.5, versus 2.5 for carbon, thus causing the carbon to be δ+ve, and the oxygen to be δ-ve. This leads to the possibility of various reactions, such as nucleophilic attack to the carbon, or electrophilic attack to the oxygen. The two main classes of carbonyls are aldehydes and ketones. The carboxyl group is also shown below.
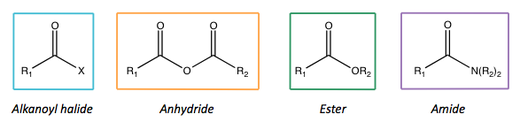
Following on from this, we can list important carboxylic acid derivatives:
| Bond | Bond Length | Bond energy |
|---|---|---|
| C-O | 1.43 Å | 351 kJmol-1 |
| C=O | 1.21 Å | 720 kJmol-1 |
Nucleophilic Addition to the Carbonyl Group
Nucleophilic Addition to Methanal
The bonding within methanal is described as follows:
Both the carbon and oxygen are sp2 hybridised, and the C-O bond is itself made of one sp2 orbital from each atom. The remaining two sp2 orbitals on each atom remain:
- as lone pairs in the case of the oxygen
- used for further bonding in the case of the carbon
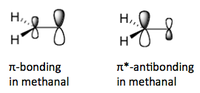
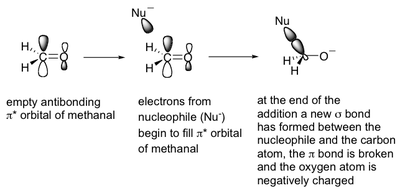
When a nucleophile approaches methanal for attack, its electrons are donated into methanal's antibonding π* orbital.
Population of an antibonding orbital results in loss of bond order from 2 to 1, which is why the C=O becomes simply C-O.
The antibonding and bonding orbitals on methanal are given below:
At the end of the nucleophile's attack, a new σ bond has formed from the carbonyl C to the nucleophile, and the O has become negatively charged, since electrons from the double bond have migrated towards it, leaving behind only a single C-O bond. All this results in the generation of a charged tetrahedral intermediate.
Maximum overlap between the nucleophile and methanal's antibonding orbital happens when the nucleophiles is perpendicular to the carbon atom.
However, the electrostatic repulsion supplied by methanal's filled bonding orbital causes the nucleophile to have to attach the carbon at an angle.
This is called the Bürgi-Dunitz trajectory, a topic covered more in depth in the 2nd year "Organic Synthesis Part 1b".
The Bürgi-Dunitz trajectory is when the nucleophile attacks specifically at an angle of 107°.
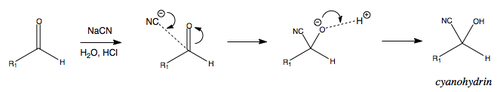
Nucleophilic Attack by Cyanide
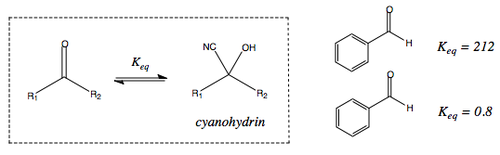
Nucleophilic attack by cyanide leads to the formation of cyanohydrins. This is a reversible process that is dependent on the structure of the starting carbonyl.
The following diagram describes the way in which varying groups on the carbonyl affect the equilibrium.
The phenyl group helps direct the attack of the nucleophile in a specific direction.
Having a hydrogen as the other R group allows for this directed attack to take place easily.
As soon as a methyl or ethyl group is in place, there is steric hindrance to the directed attack.
FORWARD REACTION
In the forward synthesis, one of the driving forces is the formation of NaCl.
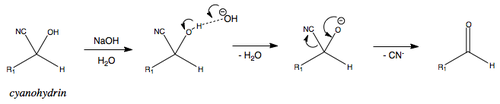
BACK REACTION
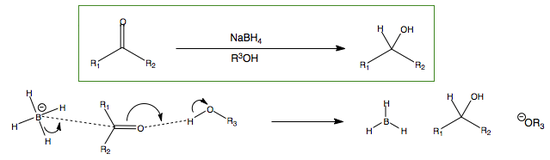
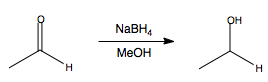
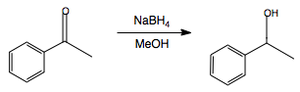
NaBH4: Reduction of Aldehydes and Ketones
It reduces a carbonyl compound via to the following general mechanism in the presence of an alcohol:
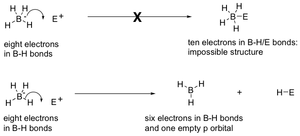
It is important to remember that sodium borohydride, NaBH4, does not attack nucleophilically directly by its negative charge. This would lead to a hypervalent compound. Instead, it attacks by donating a proton to the species it is attacking, thus changing its bonding from 4 hydrogens (8 e-) to 3 (6 e-) hydrogen atoms.
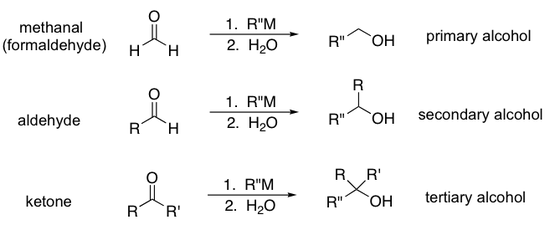
Primary and Secondary Alcohol Synthesis
Aldehydes can be reduced to primary alcohols.
Ketones can be reduced to secondary alcohols.
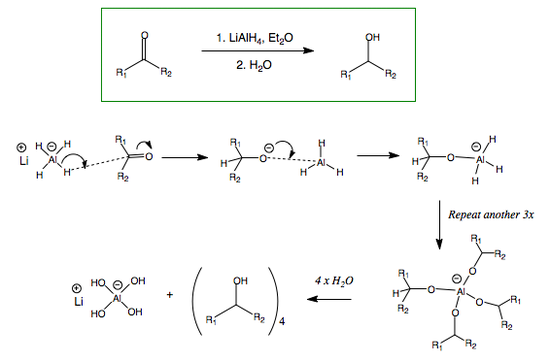
Lithium Aluminium Hydride as a Reducing Agent
Lithium aluminium hydride (LAH or LithAl) is a powerful reducing agent, used maily for the reduction of esters, carboxylic derivatives and amides. It is a pyrrophoric compound, as it reacts violently with water, releasing H2 in the process. It reduces carbonyl compounds according to the following general formula, also in the presence of an alcohol (NOT WATER - it will react, but creating hydrogen gas):
Primary and Secondary Alcohol Synthesis
Reduction of aldehyde results in primary alcohol.
Reduction of ketone results in secondary alcohol.
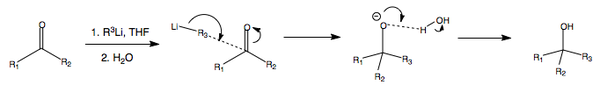
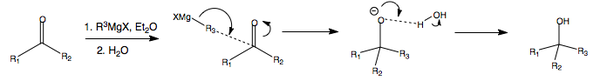
Organometallic Compounds as Reducing Agents
As an overview...
Organometallics are very useful within umpolung chemistry, where you reverse the natural polarity of a particular group. In this case, the alkyl chain's natural polarity would be to adopt a positive charge where possible, but the use of organometallics reverses this polarity. In general, organometallics such as the organolithium and organomagnesium will perform the following alcohol transformations.
ORGANOLITHIUM REDUCTION MECHANISM
GRIGNARD REAGENTS REDUCTION MECHANISM
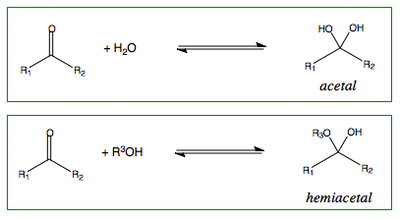
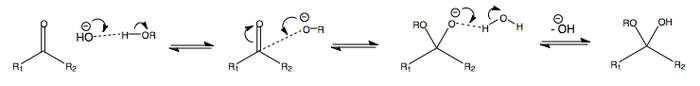
Addition of Water or Alcohols to Carbonyl Compounds
Addition of water to a ketone or aldehyde will produce an acetal.
Addition of an alcohol to a ketone or aldehyde will produce a hemiacetal.
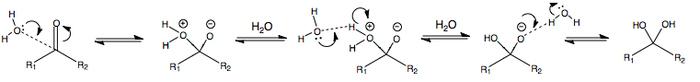
ADDITION OF WATER AND ALCOHOL AT pH7
Addition of water - formation of an acetal:
Addition of an alcohol - formation of a hemiacetal:
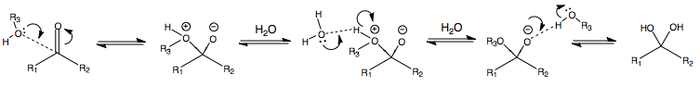
ADDITION OF WATER OR ALCOHOL AT pH < 7
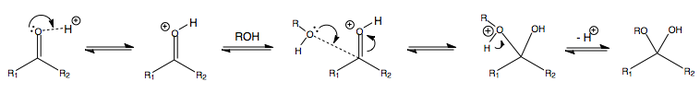
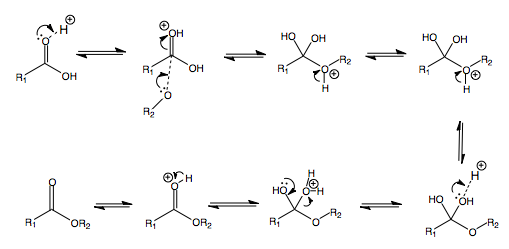
The following is the addition of water or alcohol in acidic conditions, i.e. at pH < 7.
The protonation at the start renders the carbonyl more electrophilic. This makes the subsequent addition of ROH easier.
When R = H (in the case of water), an acetal is made.
When R = alkyl (in the case of an alcohol), a hemiacetal is made.
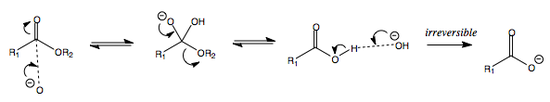
ADDITION OF WATER OR ALCOHOL AT pH > 7
The following is the addition of water or alcohol in basic conditions, i.e. at pH > 7.
The deprotonation at the beginning renders the alcohol or water anion more nucleophilic, thus facilitating nucleophilic attack.
When R = H (in the case of water), an acetal is made.
When R = alkyl (in the case of alcohol), a hemiacetal is made.
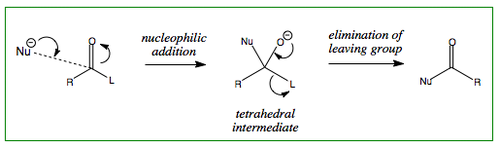
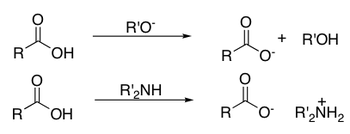
Nucleophilic Substitution at the Carboxyl Group
As described in the previous section, a C=O double bond is a region of fairly high electron density that is polarised towards the oxygen atom. Nucleophilic substitution starts i the same way as nucleophilic addition, where a nucleophile attacks the carbonyl C, but the tetrahedral intermediate then collapses back to form a trigonal planar molecule as a good leaving group is eliminated.The general mechanism for a nucleophilic substitution is shown below:
where L = leaving group
where Nu = nucleophile
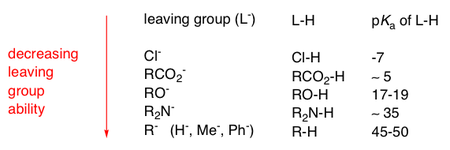
An important aspect to keep in mind is how stable the leaving group (LG) is that dissociates and it is therefore always necessary to look at the pKa of the leaving group.
The pKa of the L-H is the pH as which L-H is half dissociated, and where the LG exists as L- (i.e. the anionic state it's in when it has been eliminated).
The pKa is therefore a good guide for determining the leaving group ability.
The following table summarises a few important LG pKa/abilities:
The higher the pKa of the L-H group, the less likely it is for L-H to dissociate easily. This means it is much more difficult for L- to exist as a free anion, which decreases the leaving group ability of that particular LG. It is therefore less likely to be eliminated, unless different reaction conditions are introduced.
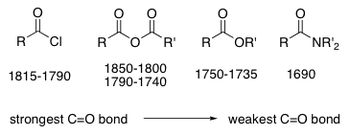
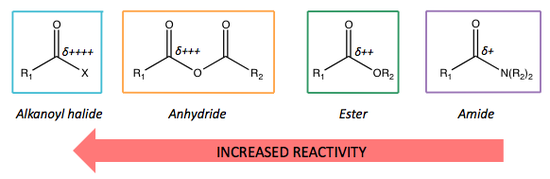
Reactivity of Carboxylic Acid Derivatives
One of the main factores that affects the reactivity of carboxylic acid derivatives is the electrophilicity of the carbonyl carbon atom.
If we consider the C=O stretching frequencies of certain carboxylic acid derivatives, we can identify which C=O bonds are the strongest and which are the weakest.
Recalling the theory learnt about IR spectroscopy, the weaker the bond, the lower the frequency at which is will vibrate and therefore the lower the wavenumber it will exhibit.
This physical data reflects the extent of delocalisation present within a molecule.
The greater the extent of delocalisation, the weaker the leaving group ability because is lessens the partial positive charge on the carbonyl carbon.
Overall, we can summarise this as:
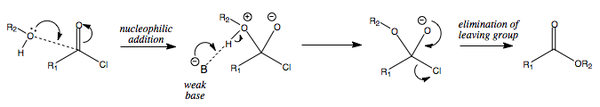
Alkanoyl Chloride + Alcohol = Ester
The reaction of an alkanoyl chloride and an alcohol leads to the formation of an ester.
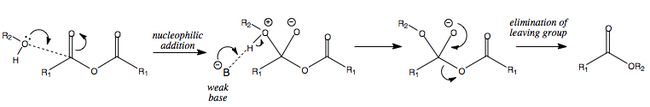
Anhydride + Alcohol = Ester
The reaction of an anhydride and an alcohol leads to the formation of an ester.
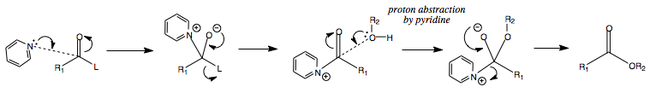
Pyridine as a Base and as a Catalyst
Following on from the two previous mechanisms, it is worth mentioning that pyridine is an excellent addition to these reactions.
As well as being a weak base, it is able to add nucleophilically to the carbonyl bond, thereby making a highly electrophilic tetrahedral intermediate. This is then able to react with the alcohol and release the leaving group.
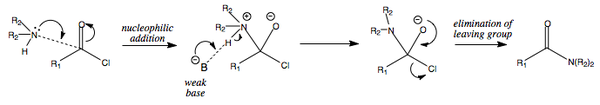
Alkanoyl Chloride + Amine = Amide
The reaction of an alkanoyl chloride and an amine leads to the formation of an amide.
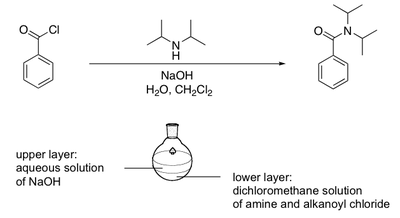
The Schotten-Baumann synthesis of amides
The reaction between an alkanoyl chloride and an amine results in the formation of an amide and one equivalent of HCl. This acid needs to be neutralised by the addition of a second equivalent of amine, or alternatively by a base such as NaOH. The Schotten-Baumann method is very useful because it separates the phases in which the amide synthesis and acid neutralisation are taking place. The amide synthesis takes place in dichloromethane (DCM, CH2Cl2) while the acide neutralisation takes place in an aqueous phase. The paper in which this method was developed was first published in 1884. The diagram below summarises this process:
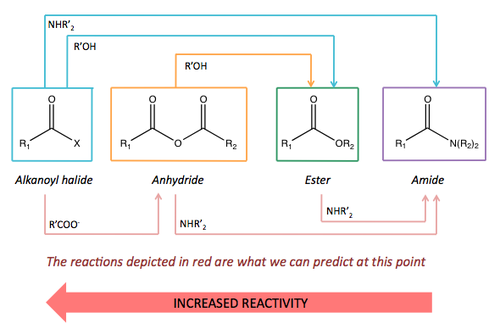
Summary of Carboxylic Acid Derivative Reactivity
Below is the list of carboxylic acid derivative syntheses and the interconversions covered so far.
From this, it is possible to predict the last three interconversions based on the knowledge we have.
The reason why this order of reactivity exists and why an amide can't be reacted with an alcohol, for example, to achieve an ester is to do once again with pKa.
When going from an ester to an amide and from an amide to an ester, the tetrahedral intermediate formed would be exactly the same. ROH has a lower pKa that NR3, which means -OR is more stable when eliminated as the leaving group, than -NR2. The follwing diagram and table summarise this.
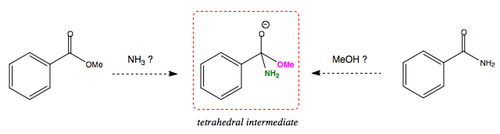
| LG | pKa | Does it leave the Td intermediate? |
|---|---|---|
| Ph- | 45 | Nope. Chuck Testa. |
| NH2- | 35 | Nope. |
| MeO- | 16 | YES |
Having ordered all carboxylic acid derivatives in order of reactivity, it becomes evident that there is no intervconversion from any of the other derivatives to alkanoyl halides.
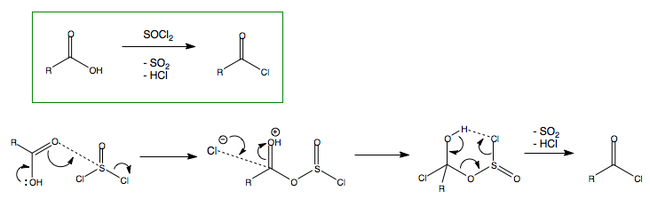
The way in which to obtain alkanoyl chloride is as follows:
Reactivity of Carboxylic Acids
Where do carboxylic acids fall in the reactivity order of their derivatives?
Hydrolysis of carboxylic acid derivatives supports the idea that carboxylic acids lie just above esters in terms of reactivity. While alkanoyl halides react fast with water at 20 °C and anhydrides react fairly slowly with water at 20 °C, there absolutely no reaction between esters or amides with water under the same conditions. This places acids just above esters in terms of reactivity.
However, problems arise when trying to convert carboxylic acids to ester or amides under neutral or basic conditions.
Instead, esters must be synthesised from carboxylic acids by means of acid catalysis.
Amides must be synthesised from carboxylic acids using DCC (dicyclohexylcarbodiimide).
Esters from Carboxylic Acids
The Acid-Catalysed Synthesis of Esters
The equilibrium constant for this reaction is generally about 1, but different conditions can help drive the equilibrium right.
- Adding excess alcohol can help drive the reaction to the left, as the reaction strives to lessen the concentration of alcohol.
- Adding a drying agent, such as silica gel, removes the water produced by the reaction and therefore drives the equilibrium right.
The Base-Catalysed Ester Hydrolysis
The last step of this hydrolysis is irreversible. This is due to the pKa of the carboxylic acid being around 5 compared to the pKa of water, which is 16.
This means that the base-catalysed synthesis of esters is impossible. It should also be noted that althougb this hydrolysis is base-catalysed, the carboxylate anion generated at the end consumes one equivalent of base.
Once again, the reaction can be driven right by changing the conditions.
For example, adding excess water can drive the hydrolysis forward.
Amides from Carboxylic Acids
The Synthesis of Amides using DCC
The reason why this synthesis works so well is because the DCC (dicyclohexylcarbodiimide) activates the carboxylic acid in situ, making it more electrophilic.
Acid-Catalysed Amide Hydrolysis
The formation of the ammonium salt is irreversible, which is what makes the entire hydrolysis irreversible.
Base-Catalysed Amide Hydrolysis
The last step is irreversible due to, as has been mentioned, the pKa of the final carboxylate.
Final Summary of Carboxylic Acid Derivative Reactivity
This is a slightly crazy flow chart, so no need to memorise it or anything, it's just a summary of all the carboxylic acid derivative interconversions covered in this section.
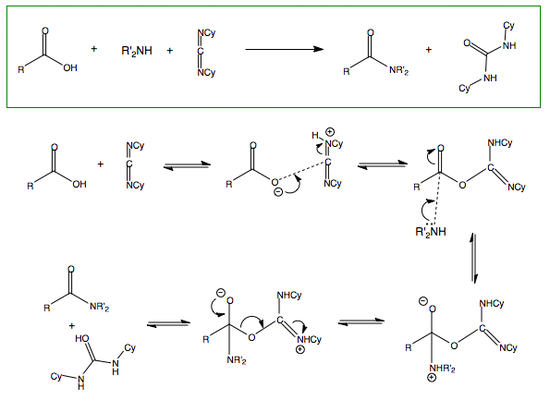
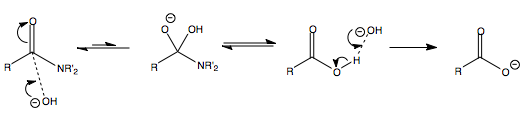
Nucleophilic Substitution at the Carbonyl Group with Loss of Carbonyl Oxygen
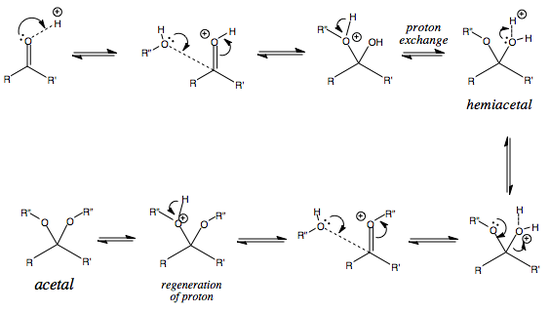
Carbonyl Interconversion to Hemiactetal and Acetal
The mechanism for the formation of acetals and hemiacetals is given below.
The problem with this acetal formation equilibrium is that three molecules (carbonyl plus two ROH molecules) are converted to two molecules (acetal and water).
This is entropically unfavourable, which means that the equilibrium lies quite significantly to the left. This problem has two solutions, both of which exploit the Le Chetalier principle:
- Use an excess of one of the reagents, forcing the equilibrium right
- Use a water-absorbing agent such as molecular sieves (zeolite) to remove water from the reaction mixture.
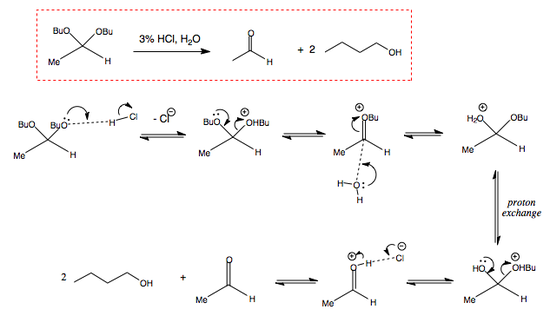
ACID-CATALYSED HYDROLYSIS OF ACETAL
Acetals as Carbonyl Protectors
One very useful application of acetals is for the protection of carbonyl groups. A reaction can then be performed on another functional group in the molecule, followed by a deprotection of the initial protected carbonyl, thus leaving it unchanged. This protection of carbonyls is usually accomplished by using a diol (instead of two separate alcohol molecules) and results in the creatoi of a "cyclic" acetals that crate an alkyl "bridge" in place of the protected carbonyl. The following mechanism will demonstrate:
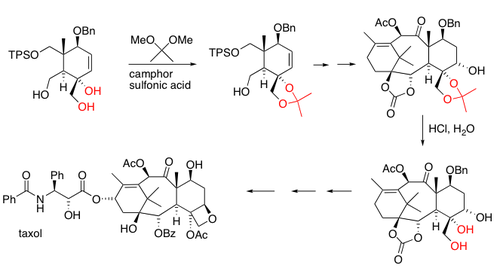
Here is an example of acetal protection used in the synthesis of much larger and complicated molecules.
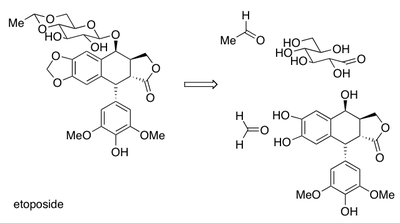
It is also useful to be able to identify acetal linkages. Etoposide is an anti-cancer drug that contains three acetal linkages.
Carbonyl Interconversion to Hemiaminal and Imine
The reaction between a carbonyl and a primary amine yields an imine.
The reaction between a carbonyl and a secondary amine yields an enamine.
Imine Formation
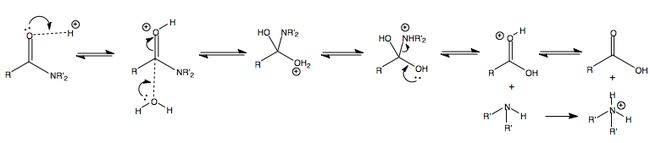
The formation of an imine proceeds in much the same way as the firmation of a hemiacetal and acetal. The amine attacks and adds nucleophilically to the carbonyl, followed by proton echange and the release of water to yield an imine. The mechanism is displayed below.
The rate of imine formation is in fact pH dependent. The maximum rate occurs at a specific pH for a particular carbonyl and particular amine, but generally lies in the region of pH 4-6.
Imine formations is at the core of Nature's way of synthesising and degrading amino acids such as alanine. The equilibrium position is controlled by enzymes.