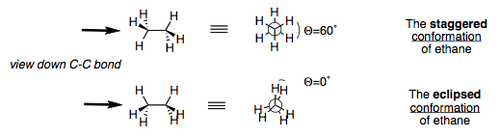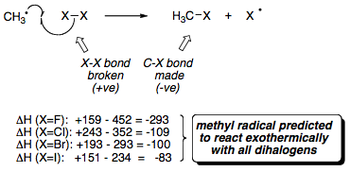StudentWiki:AlkanesAlkenesAlkynes
Alkanes, Alkenes, Alkynes (AAA)
The contents of this page are taken from Dr. Chris Braddock's lecture course (2010-2011) and are copyright of Imperial College London
Introduction
This course is an introduction to the basic nomenclature, structures and physical properties of alkanes, alkenes and alkynes.
Alkanes
Names, Nomenclature, Structures
The naming of alkanes follows the same trend as that of alkenes and alkynes. Basically, any hydrocarbon chain follows the same naming process according to the number of carbons. The prefix (meth-, eth-, prop-, but-, etc) determines the number of carbons present, and the ending describes the type of molecule, "-ane" in this case denoting an alkane. Alkanes are an important class of compounds. They are immiscible with water due to their being non polar (whereas water is polar) and have a general density of approximately 0.8 gcm-3, meaning that they will float on water.
- Linear alkanes follow the general formula = CnH2n+2
- Cyclic alkanes follow the general formula = CnH2n

| State at R.T. | Carbon number n |
Name | Chemical Formula |
Melting Point (°C) |
Boiling Point (°C) |
|---|---|---|---|---|---|
| GAS | 1 | methane | CH4 | -183 | -162 |
| 2 | ethane | C2H6 | -172 | -89 | |
| 3 | propane | C3H8 | -187 | -42 | |
| 4 | butane | C4H10 | -138 | 0 | |
| LIQUID | 5 | pentane | C5H12 | -130 | 36 |
| 6 | hexane | C6H14 | -95 | 69 | |
| 7 | heptane | C7H16 | -90.5 | 98 | |
| 8 | octane | C8H18 | -57 | 126 | |
| 9 | nonane | C9H20 | -54 | 151 | |
| 10 | decane | C10H22 | -30 | 174 | |
| 11 | undecane | C11H24 | -26 | 196 | |
| 12 | dodecane | C12H26 | -10 | 216 | |
| 13 | tridecane | C13H28 | -6 | 234 | |
| 14 | tetradecane | C14H30 | 5.5 | 252 | |
| 15 | pentadecane | C15H32 | 10 | 266 | |
| 16 | hexadecane | C16H34 | 18 | 280 | |
| 17 | heptadecane | C17H36 | 22 | 292 | |
| SOLID | 18 | octadecane | C18H38 | 28 | 308 |
| 19 | nonadecane | C19H40 | 32 | 320 | |
| 20 | icosane | C20H42 | 36 | - |
The explanation as to why the melting points and boiling points increase so steadily as the hydrocarbon chain lengthens is due to the increased molecular weight of the molecule. The longer it is, the more intermolecular Van der Waal forces are felt, thus holding the linear chains together more effectively and increasing the amount of input energy necessary to cause the substance to melt and eventually boil.
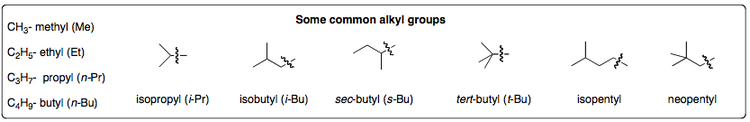
Other non-linear unsaturated hydrocarbons exist as well, such as cyclic alkanes (displayed above) and other alkyl groups. These both have important applications within organic synthesis as building blocks for other more complex molecules and also as protecting groups, i.e. for shielding a particular functional group when targeting another one. The most common alkyl groups are given below:
Rules for naming branches alkanes (that are also applicable to linear alkanes):
1. A saturated branched hydrocarbon is named by prefixing the designations of the side chains to the name of the longest chain.
2. The longest chain is numbered from one end to the other, the direction being chosen so as to give the lowest numbers possible to the side chains.
3. If two or more chains of different nature are present they are cited in alphabetical order.
4. The presence of identical substituents may be indicated by the appropriate multiplying prefix di, tri, tetra, penta, hexa, etc.
For example:
Structure of Methane
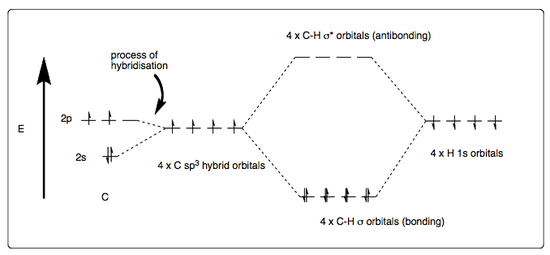
Methane is a tetrahedral molecule. The simplest way of approaching its bonding model is to consider its hybridisation model as the overlap of carbon's single s-orbital with its three p-orbitals, thus forming four degenerate sp3 orbitals. These are then capable of combining (overlapping) with four hydrogen s-orbitals and generating four C-H σ bonds. This means all bond lengths are of equal length and electron density, causing methane to adopt its tetrahedral geometry, with bond angles of 109°28'. Its bond lengths are al the same at 1.09 Å, with a bond energy of 435 kJmol-1 each. The following diagrams summarise this paragraph.
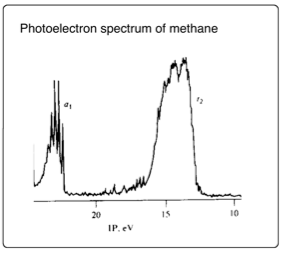
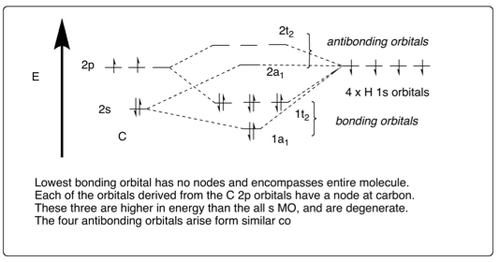
Nevertheless, the spectral data obtained for methane shows two absorbance peaks instead of the expected single peak (according to the model proposed in the previous paragraph). This implies that the four C-H σ bonding orbitals are not degenerate and that in fact carbon's s- and p-orbitals combine directly with the four degenerate hydrogen s-orbitals, producing four bonding orbitals of almost indistinguishable energy, thereby accounting for the perfect tetrahedral geometry. The populated orbital of a1 symmetry nevertheless lies slightly lower in energy and therefor produces a different spectral peak to the rest.
Structure of Ethane
Similarly to methane, ethane is sp3 hybridised at each carbon atom. It contains two carbon atoms, joined together by a C-C bond, which introduces the possibility of rotation about the C-C bond, and therefore the existence of different ethane conformations. This is an extremely important concept within chemistry for it opens the doors to many different reaction pathway possibilities when discussing the conformations of higher molecules. Ethane is a basic molecule, that has two main conformations: staggered and eclipsed.
These are useful representations of ethane. The diagrams to the left are called "sawhorse" projections, and those to the right are alled Newman projections. Newman projections are useful for representing different conformations because they display the relative orientation of different groups on the molecule when looking "down" the C-C bond.
The energy profile for ethane is as follows for a complete 360° rotation about the C-C bond.
The staggered conformation is the one with the greatest stability and therefore the lowest energy. This is due to the even distribution of the hydrogen atoms. The eclipsed conformation has the hydrogen atoms much closer to each other, therefore causing the greatest steric hindrance to each other and generating the greatest torsional strain. Of course, ethane is still a simple molecule, so the barrier for rotation due to torsional strain is much smaller in comparison to butane, for example, and other larger molecules.
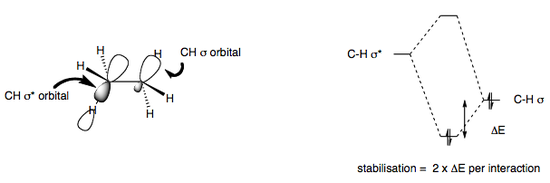
In fact, for ethane the steric hindrance caused by two approaching hydrogen atoms hardly accounts for the torisional strain of the molecule (only about 10%). Another contributor can arguably be the repulsions between electrons in bonds of close proximity, but again this is only a minor contribution to torsional strain. A better argument is that there exists a stabilising interaction between C-H σ and C-H σ* orbitals that is only possible in the staggered conformation, thus increasing the torsional strain when achieving an eclipsed conformation. This stabilising interaction is also present in the conformational analysis of other higher molecules, but obviously steric factors take on greater importance.
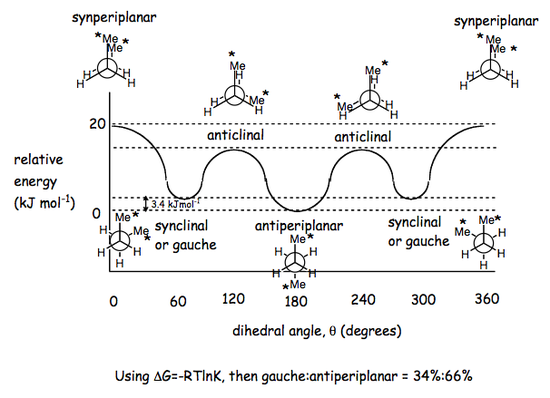
Structure of Butane
Butane introduces a greater complexity when analysing its conformations. Once again, staggered conformations are the most stable, but the one with the lowest energy is the one in which the methyl groups either end of the central C-C bond are located as far away from each other as possible, i.e. perfectly trans to each other within the antiperiplanar conformation. A staggered conformation in which the methyl groups are at a 60° tilt to each other is called synclinal (or gauche) and is less stable that the antiperiplanar one. The eclipsed conformations are the least stable, especially the one where the Me groups are perfectly cis to each other, i.e. at 0° to each other. This synperiplanar structure holds the greatest steric hindrance, and that encompasses the largest torsional strain. Eclipsed conformations where the methyl groups are at roughly 120° to each other are called anticlinal.
Structure of Cyclohexane
Cyclohexane is a 6-membered hydrocarbon ring that usually exists in a chair conformation. This conformation is advantageous because of a total absence of ring strain thanks to all bonds being sp3 with bond angles of 109.5°, i.e. all bonds exist in a completely staggered environment. As you can see from the diagram below, there is one environment for all carbon atoms and two different environments for the hydrogen atoms: the axial and the equatorial orientations.
The 13C spectrum of a chair conformation of cyclohexane displays only one signal (as expected) at 25.2 ppm. The 1H spectrum, on the other hand only displays one signal at 1.40 ppm, which seems to contradict the existence of the two orientations. This is due to "ring flipping", a process that interconverts axial and equatorial hydrides. It happens so fast that only a single average signal is visible at room temperature. This equilibrium process can be slowed down by reducing the temperature so that the two different isomers are detected in the 1H NMR spectrum.
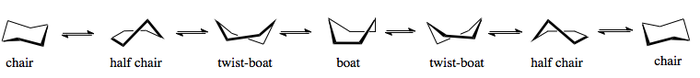
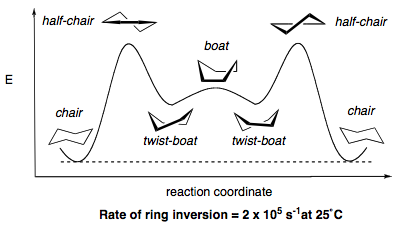
The entire ring inversion can be expanded into the following equilibrium.
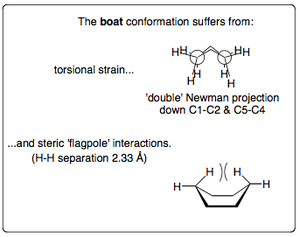
The boat conformation is another important conformation of cyclohexane. It is not as stable as the chair conformation because all of the C-H bonds are eclipsed rather than staggered. Furthermore, a steric hindrance that comes into effect as the two hydrides in the "flagstaff" (or "flagpole") positions come into close proximity of each other - roughly 2.33 Å. This explains why the boat conformation isn't adopted nearly as readily, even though there is no actual angular strain within the C-C bonds.
The conformational analysis of cyclohexane is provided below. The boat conformation is approximately 25 kJmol-1 higher in energy than the chair conformation, although the most unstable structure of all is the half chair conformation, with a torsional strain barrier of 43 kJmol-1. The rate of ring inversion is overall about 2 x 10-5 s-1.
Substituted Cyclohexanes
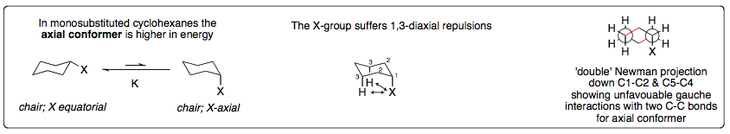
A monosubstituted cyclohexane molecule has two conformational options - either the substituent is in the axial position or the equatorial one. The axial one is the structure of highest energy (greatest instability), which means the equilibrium lies largely towards the equatorial structure. This means at any given time there will be a lesser concentration of he axial-substituted compound.
The equatorial conformation is more stable due to two important factors. The first is the lack of 1,3-diaxial repulsions, also known as the 1,3-diaxial interaction. These exist between an axial substituent and the two other hydrides that lie along the same plane (in other words, the hydrides on the same side of the ring). The second contributor is the fact that an equatorial substituent is antiperiplanar to two C-C bonds, whereas an axial substituent is gauche to two C-C bonds.
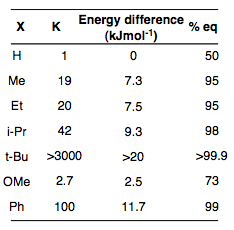
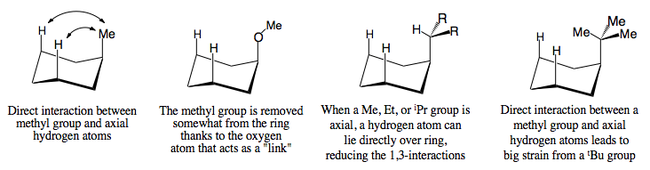
The table opposite describes the effect that increasing size has on the equilibrium between axial and equatorial conformations for a monosubstituted cyclohexane. Obviously as the bulk of an alkyl group increases, it is more likely to exist in the equatorial position as the barrier to a conversion to the axial conformation becomes more significant. Nevertheless, steric factors are not actually the most important point to consider. Rather, an analysis of the interactions between the group and the proximal hydrides will give a better idea of why the methoxy group, for example has a much larger proportion of axial substituent at any given time.
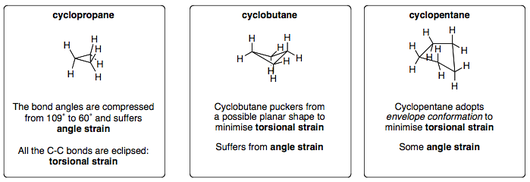
Smaller Cycloalkanes
Smaller cyclohexanes must endure angular train as well as torsional strain and are therefore less stable than cyclohexane.
Alkane Reactivity
Halogenation of Methane
Methane is capable of reacting with halogens besides chlorine: fluorine and bromine.
FLUORINATION
When methane reacts with fluorinem the reaction is so vigorous and explosive that it cannot be performed in a standard laboratory. This is due to the formation of C-F bonds that are so strong that the heat released during the reaction is too dangerous. Carbon and fluorine orbitals have very similar energies and are therefore capable of excellent overlap with each other, hence the strength of the bonds generated.
CHLORINATION
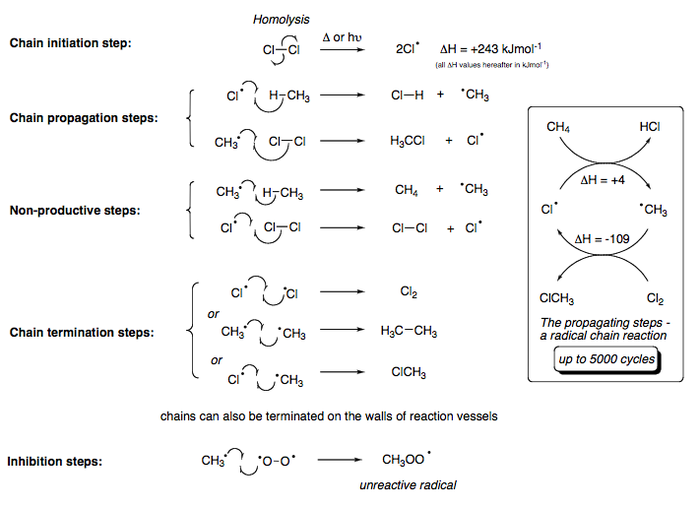
Once again, chlorine orbitals are of advantageous size and energy for a successful overlap with carbon's orbtials, thus producing a fast reaction (although much more manageable that the reaction with fluorine). The chlorination of methane proceeds via a free-radical chain mechanism that is described in further detail within the next section. The general outline for the reaction is given below.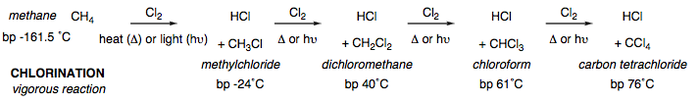
BROMINATION
The bromination is a smooth reaction, significantly slower than the reaction with chlorine or fluorine. This is due to bromine being a rather heavy atom with diffuse s-orbitals that are less capable of overlapping with carbon's sp3 orbitals. Bromination occurs in much the same fashion as the chlorination of methane; the general mechanism is given below.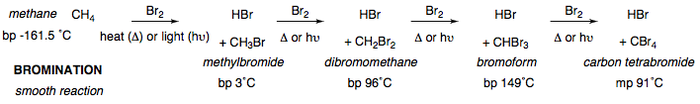
IODINATION
No reaction at all takes place between methane and iodine, due to the unfavourable overlap of atomic orbitals.
Iodine is so diffuse that no positive interaction can be accomplished, thereby accounting for the lack of reaction.
Chlorination of Methane
What is the mechanism for these reactions and how does it explain reactivity? Some facts:
- Methane and chlorine do not react in the dark at room temperature.
- The reaction takes place readily in the dark at temperatures over 250˚C or under UV radiation.
- The wavelength of light that induces chlorination is that known independently to cause dissociation of chlorine molecules.
- In the light-induced reaction, several thousand molecules of methyl chloride are obtained for each photon of light that is absorbed.
- The presence of a small amount of oxygen slows down the reaction for a period of time, after which the reaction proceeds normally; the length of this period depends upon how much oxygen is present.
The chlorination of methane proceeds via a free-radical chain reaction, initiated by the presence of UV radiation. The mechanism is as follows:
Reactivity of Halogens Towards Methane
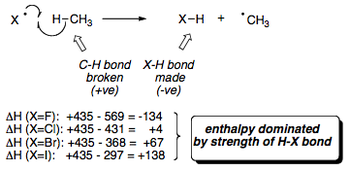
Following on from the discussions in the previous sections, the diagram below displays the individual bond energies for X-X, H-X and H3C-X bonds (where X = halogen). This data clearly displays the increased bond strength of a C-F bond in methane compared to a normal C-H bond and all other halogenated methane molecules.
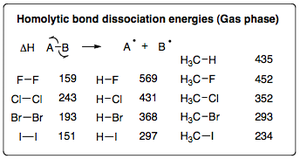
Looking at the X2 bond energies in particular, it is interesting to note that the cleavage of the I2 bond doesn't in fact require much energy, which you would think would cause the rate of reaction for the iodination of methane to increase compared to that of the chlorination, but this is not the case. A more in depth analysis has to be made.
Activation energy of >138 kJmol-1 for iodine atoms means that they are unable to abstract a hydrogen atom from methane and carry the radical chain (even though it is relatively easy to generate Iodine atoms from I2). Ultimately, the reactivity of a halogen towards methane depends upon the strength of the bond which that halogen forms with hydrogen.
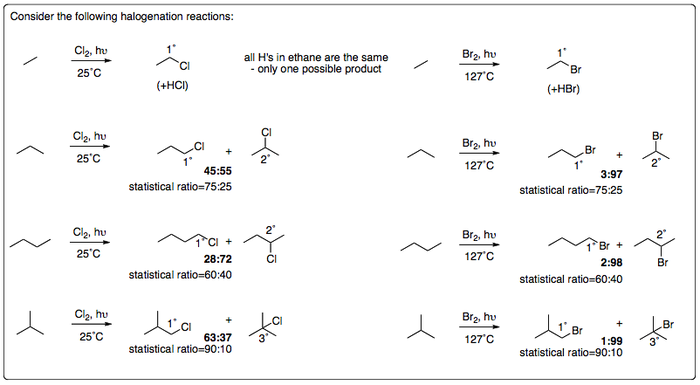
Reactivity of Halogens Towards Alkanes
Halogenation of other alkanes follows the same mechanism as per methane, but multiple products can obviously result.
CONCLUSIONS
Product distributions show a preference for 3° > 2° > 1° products.
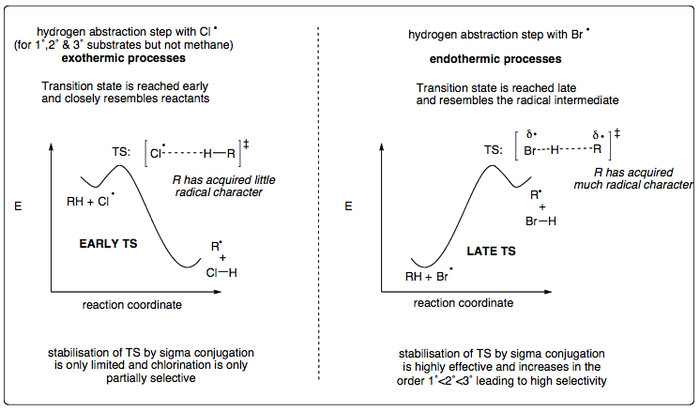
Brominations are more selective than chlorinations (even at elevated temperatures). This can be explained by an understanding of the order of stability of radicals and by involving the concept of Hammond's postulate.
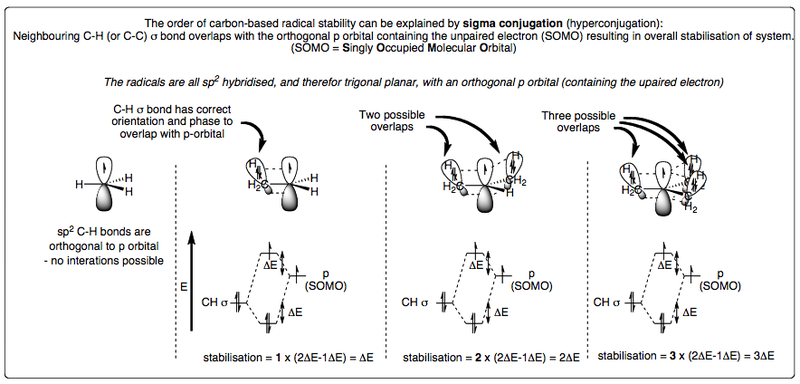
Stability of Radicals - Sigma Conjugation
A methyl radical is less stable that a primary one, which is less stable than a secondary one, which is less stable than a tertiary one.
In other words Me < 1° < 2° < 3°
Hammond's Postulate
Hammond's Postulate (also known as the Hammond-Leffler postulate) is a hypothesis that says:
"If two states, for example, a transition state and an unstable intermediate, occur consecutively during a reaction process and have nearly the same energy content, their interconversion will involve only a small reorganisation of the molecular structures."
The original paper may be found here DOI:10.1021/ja01607a027