Sb1016 reportYr1
NH3 Analysis
test molecule |
After optimisation, NH3 bond length was found to be 1.01798 Å and the bond angles between the nitrogen atom and the hydrogen atoms were 105.745°. It can also be seen that in Ammonia, Nitrogen has a charge of -1.125 whereas the three hydrogen atoms each has a charge of 0.375. These results are as per expectations as Nitrogen is a more electronegative atom than hydrogen and therefore, draws the electron cloud towards itself. Therefore has a more negative charge than hydrogen.
Here is a summary of the result from the optimisation:
| Calculation Method | RB3LYP |
| Basic Set | 6-31G(d,p) |
| Final Energy E(RB3LYP) | -56.55776873 au |
| RMS gradient | 0.00000323 |
| Point Group | C3V |
This optimisation has given final force and displacement values within the threshold values. This shows that the data was truly converged.
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000006 | 0.000450 | YES |
| RMS Force | 0.000004 | 0.000300 | YES |
| Maximum Displacement | 0.000014 | 0.001800 | YES |
| RMS Displacement | 0.000009 | 0.001200 | YES |
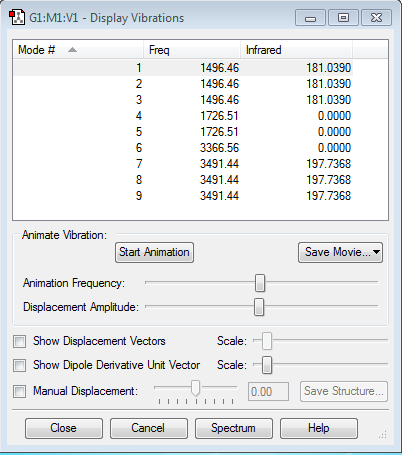
Vibrational Analysis of NH3
As NH3 is a molecule with four atoms, according to the 3N-6 rule, 6 modes of vibrational frequencies are expected.
The results from the analysis with the respective modes of frequency is given below:
It can be observed that the vibrational modes 2 and 3 are degenerate while 5 and 6 are degrease too. These modes are the same frequencies and therefore the same energy. Modes 1,2 and 3 are bending modes whereas modes 4,5, and 6 are stretching modes. Stretching modes have higher energy than the bending mode. However as there are 2 set of degenerate modes, only four bands can be identifies from the experimental spectrum of Ammonia. Another key observation is that mode 4 is highly symmetric where as mode 1 resembles an umbrella. It can be seen that there are no negative frequencies.
H2 Analysis
test molecule |
The results summary from the optimisation is given below:
| Calculation Method | RB3LYP |
| Basic Set | 6-31G(d,p) |
| Final Energy E(RB3LYP) | -1.17853936 a.u. |
| RMS gradient | 0.00000017 a.u. |
| Point Group | D*H |
The final force and displacement can also be seen for the hydrogen molecule. The table below shows that the data is converged and is within the threshold limit.
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000000 | 0.000450 | YES |
| RMS Force | 0.000000 | 0.000300 | YES |
| Maximum Displacement | 0.000000 | 0.001800 | YES |
| RMS Displacement | 0.000001 | 0.001200 | YES |
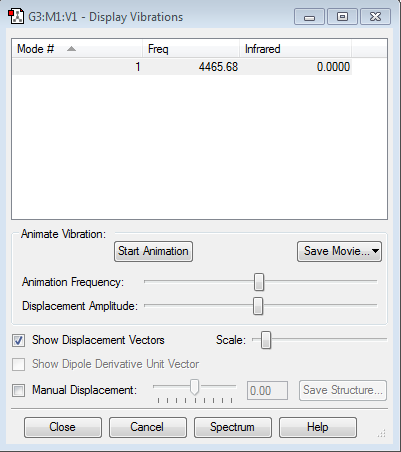
Vibrational Analysis of H2
There is only one mode of frequency as expected. This frequency is not negative and therefore acceptable. This mode is due to the stretching if the hydrogen bond.
N2 Analysis
test molecule |
The summary of the results from the optimisation is presented in the table below.
| Calculation Method | RB3LYP |
| Basic Set | 6-31G(d,p) |
| Final Energy E(RB3LYP) | -109.52412868 a.u. |
| RMS gradient | 0.00000017 a.u. |
| Point Group | D*H |
The table below give the final force and displacement of the nitrogen molecule. It shows that the data is converged and is within the threshold limit.
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000006 | 0.000450 | YES |
| RMS Force | 0.000006 | 0.000300 | YES |
| Maximum Displacement | 0.000002 | 0.001800 | YES |
| RMS Displacement | 0.000003 | 0.001200 | YES |
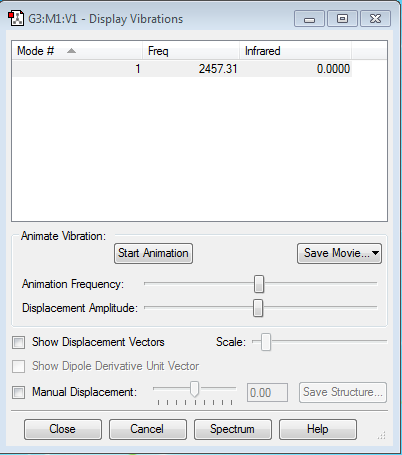
Vibrational Analysis of N2
Even with the nitrogen molecule, there is only one mode of frequency. This frequency is not negative and therefore acceptable. This mode is due to the stretching if the nitrogen bond. This frequency is higher than the vibrational frequency of the hydrogen molecule as nitrogen bond is much stronger (triple bond).
Energy Analysis of NH3
N2 + H2 → NH3
| E(NH3) | -56.55776873 au |
| 2*E(NH3) | -113.1155375 |
| E(N2) | -109.52412868 a.u. |
| E(H2) | -1.17853936 a.u. |
| 3*E(H2) | -3.53561808 a.u. |
| ΔE | -0.05579074 a.u |
| ΔE | -146.48 kJ/mol |
This an an exothermic reaction. The product is lower in energy than the reactants, therefore ammonia is more stable than nitrogen and hydrogen gas.
Formation of NH4+
NH3 + H+ → NH4+
As seen above, the nitrogen atom in NH3 has a strong negative charge, which attracts a proton. The nitrogen then donates a lone pair of electron to the H+ atom to form Ammonium ion.
NH4+ Analysis (Molecule of choice)
test molecule |
NH4+ has a bond length of 1.02761Å and the bond angle is 109.471°.
A summary of the results from the optimisation of NH4+ is given below:
| Calculation Method | RB3LYP |
| Basic Set | 6-31G(d,p) |
| Charge | +1 |
| Final Energy E(RB3LYP) | -56.90586436 a.u. |
| RMS gradient | 0.00011991 |
| Point Group | TD |
The final force and displacement values are also given below in the table. Again, the values are below the threshold limit and the data is converged.
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000232 | 0.000450 | YES |
| RMS Force | 0.000124 | 0.000300 | YES |
| Maximum Displacement | 0.000537 | 0.001800 | YES |
| RMS Displacement | 0.000287 | 0.001200 | YES |
Vibrational Analysis of NH4+
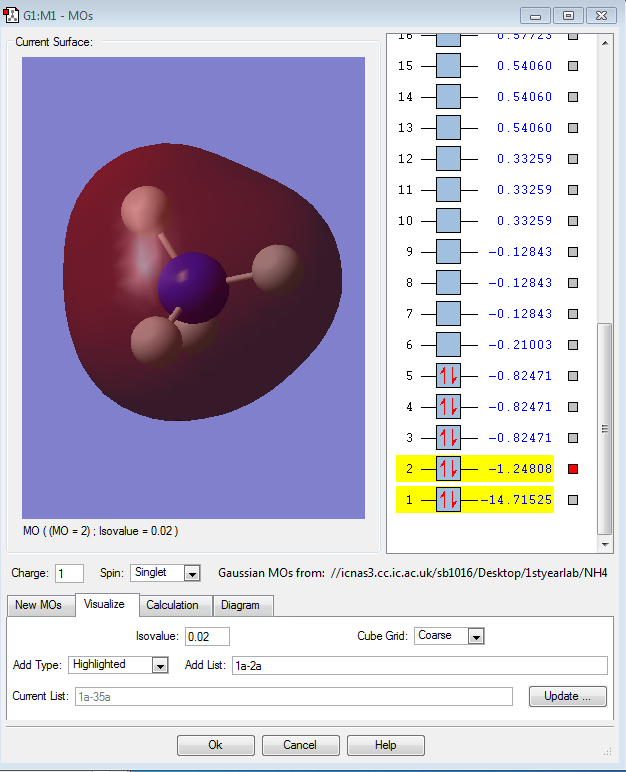
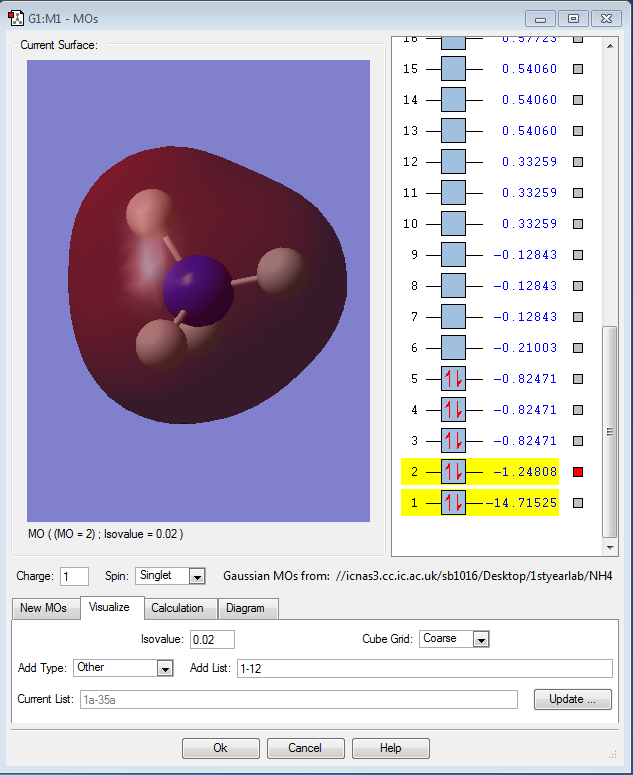
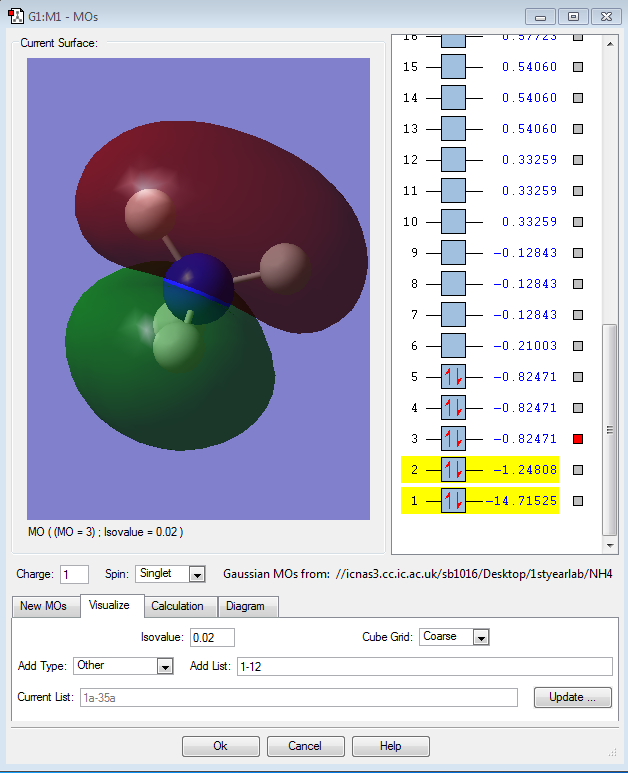
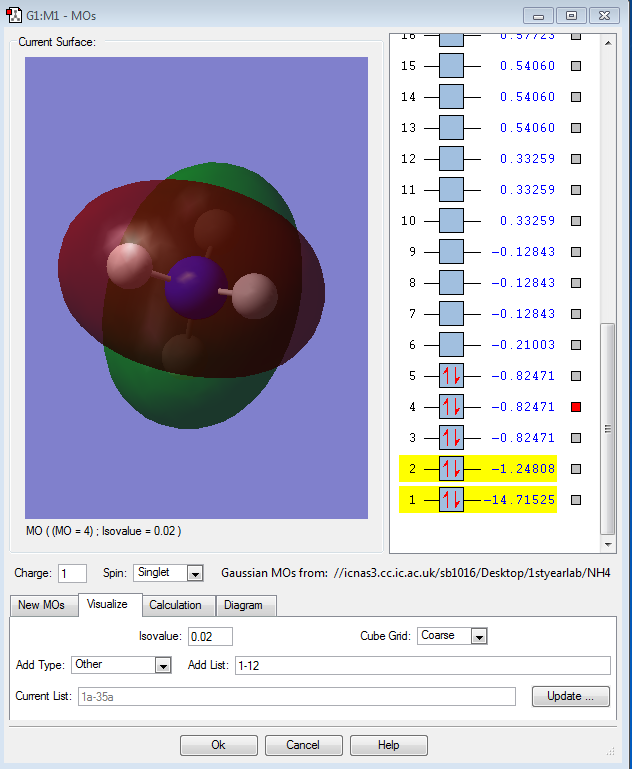
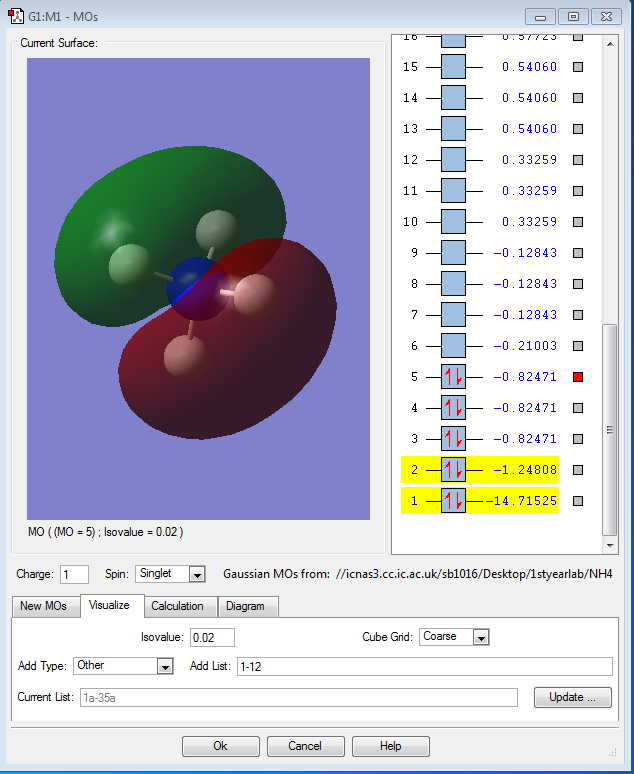
Molecular Orbital Analysis of NH4+
The first orbital is the atomic 1s non-bonding orbital of nitrogen which is very deep in energy.
The second orbital is the molecular bonding orbitl which has contributions from the 2s orbital of the nitrogen atom and the 1s orbitals of the 4 hydrogen atoms. This orbital is far higher in energy than the first orbital.
The other three orbitals are degenerate as they are the bonding orbitals due to the 3 2p orbitals of the nitrogen atoms and the 1s orbitals of the 4 hydrogen atoms. These are the HOMOs. They are the highest occupied molecular orbitals.
These MOs are not hybridised. Therefore, there are only three degenerate MOs, There should be four as N is sp3 hybridised, which is not reflected in these images. All these orbitals are occupied.
Comparison between NH4+ and NH3
| Molecule | Energy | Bond Length | Bond angle |
|---|---|---|---|
| NH3 | -56.55776873 au | 1.01798 Å | 105.745° |
| NH4+ | -56.90586436 a.u. | 1.02761Å | 109.471° |
The energy of NH3 and NH4+ are very similar. This shows that both ammonia gas and ammonium ion have similar stability. The Structure of NH3 and NH4+ are also similar. Both have a tetrahedral pseudo structure, with similar bond angle and bond lengths. This shows that nitrogen in both the molecules has the same hybridisation.