SAS
Appearance
Item Value Threshold Converged? Maximum Force 0.000161 0.000450 YES RMS Force 0.000105 0.000300 YES Maximum Displacement 0.000638 0.001800 YES RMS Displacement 0.000418 0.001200 YES
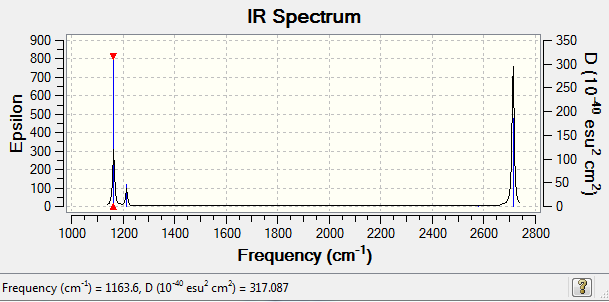
Low frequencies --- -0.2456 -0.1129 -0.0054 44.0270 45.1846 45.1853 Low frequencies --- 1163.6049 1213.5924 1213.5951
Optimised BH molecule |
Vibrational spectrum for BH3
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 92 | A2 | yes | out-of-plane bend |
| 1213 | 14 | E' | slight | in-plane bend |
| 1213 | 14 | E' | slight | in-plane bend |
| 2580 | 0 | A1' | no | symmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
There are only 3 peaks observed in the IR spectrum, as the first frequency 1163 cm-1 has a strong intensity. Both the second and third frequencies of 1213 cm-1 are degenerate and have a low intensity, so they are observed as one small peak. The fourth frequency 2580 cm-1 has no net dipole moment, so has no intensity, so is not observed. The final two frequencies at 2713 cm-1 are degenerate and have a strong intensity, so are observed as one strong signal.
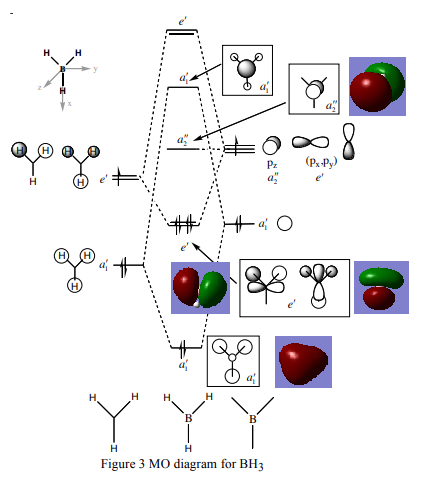
The Molecular Orbital diagram for BH3 is shown below[1]:
There are no real significant differences between the 'real' and LCAO molecular orbitals. Thus, qualitative MO theory is a very accurate representation of the real MOs. This is a useful way of visualising the real MOs.
- ↑ Molecular Orbitals in Inorganic Chemistry, Lecture 4 Tutorial Problem Model Answers, Dr. Patricia Hunt, p.2