Ruman wiki-page
EX3 section
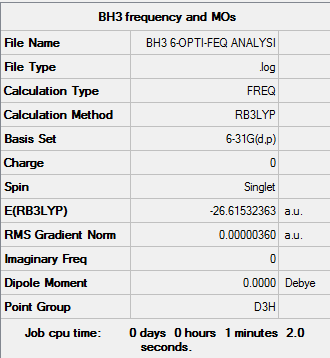
BH3
Item Value Threshold Converged? Maximum Force 0.000007 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000028 0.001800 YES RMS Displacement 0.000014 0.001200 YES
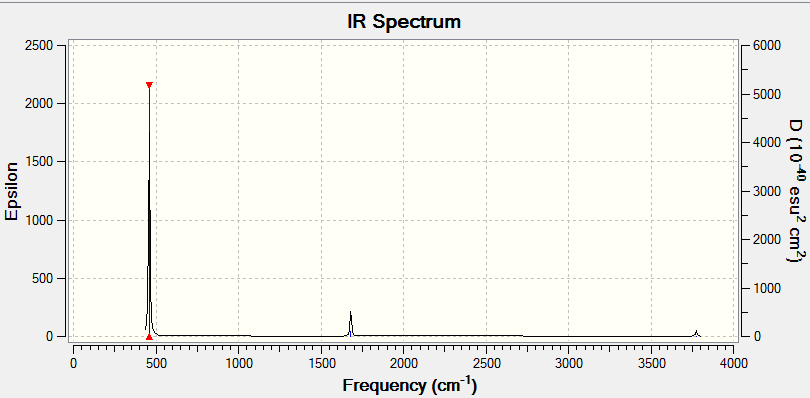
Low frequencies --- -38.8634 -38.8412 -18.7509 -0.0042 0.0451 0.2082 Low frequencies --- 458.9323 1681.0420 1681.0424
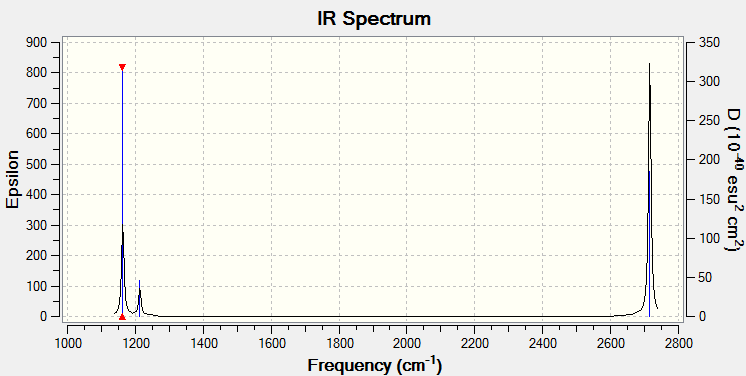
| Wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR Active? | Type |
|---|---|---|---|---|
| 1163 | 93 | A2 | Yes | out-of-plane bend |
| 1213 | 14 | E' | Very slight | bend |
| 1213 | 14 | E' | Very slight | bend |
| 2582 | 0 | A1' | No | symmetric stretch |
| 2715 | 126 | E' | Yes | asymmetric stretch |
| 2715 | 126 | E' | Yes | asymmetric stretch |
BH3 Molecule |
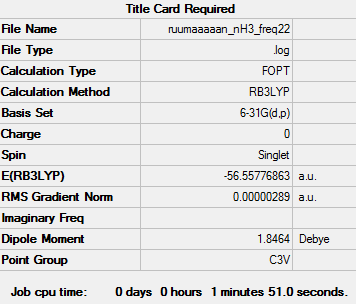
NH3
Item Value Threshold Converged? Maximum Force 0.000005 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000006 0.001200 YES
File:RUUMAAAAAN NH3 FREQIII.LOG
Low frequencies --- -11.6527 -11.6490 -0.0048 0.0332 0.1312 25.5724 Low frequencies --- 1089.6616 1694.1736 1694.1736
NH3 Molecule |
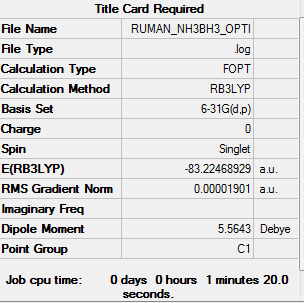
NH3BH3
Item Value Threshold Converged? Maximum Force 0.000041 0.000450 YES RMS Force 0.000014 0.000300 YES Maximum Displacement 0.000603 0.001800 YES RMS Displacement 0.000278 0.001200 YES
E(NH3)=-56.55776863
E(BH3)=-26.61532363
E(NH3BH3)=-83.22468929
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] ΔE=-83.22468929 - (-26.61532363+-56.55776863) ΔE=-0.05159au =-135kJ/mol
This energy is quite weak for a covalent bond.
Smf115 (talk) 08:10, 17 May 2018 (BST)Good consideration of the accuracy of reported energy values and correct calculation.
BH3NH3 Molecule |
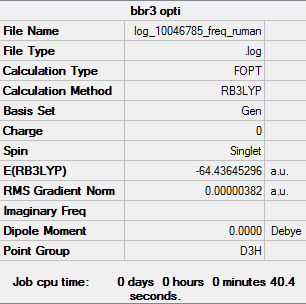
BBr3
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000023 0.001200 YES
Low frequencies --- -0.0137 -0.0064 -0.0046 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
BBr3 Molecule |
BBr3 DOI:10042/202327
Project section
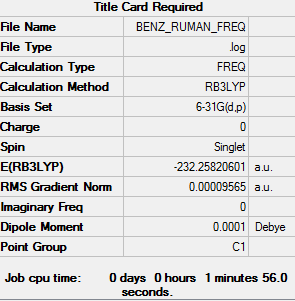
Benzene
Item Value Threshold Converged? Maximum Force 0.000196 0.000450 YES RMS Force 0.000096 0.000300 YES Maximum Displacement 0.001062 0.001800 YES RMS Displacement 0.000381 0.001200 YES
Low frequencies --- -16.9577 -14.2980 -9.2699 -0.0012 -0.0005 -0.0005 Low frequencies --- 413.7998 414.4799 620.8650
Benzene |
Borazine
Item Value Threshold Converged? Maximum Force 0.000117 0.000450 YES RMS Force 0.000036 0.000300 YES Maximum Displacement 0.000327 0.001800 YES RMS Displacement 0.000104 0.001200 YES
Low frequencies --- -11.3791 0.0008 0.0011 0.0014 9.0995 10.8730 Low frequencies --- 288.5041 290.3971 404.0139
Borazine |
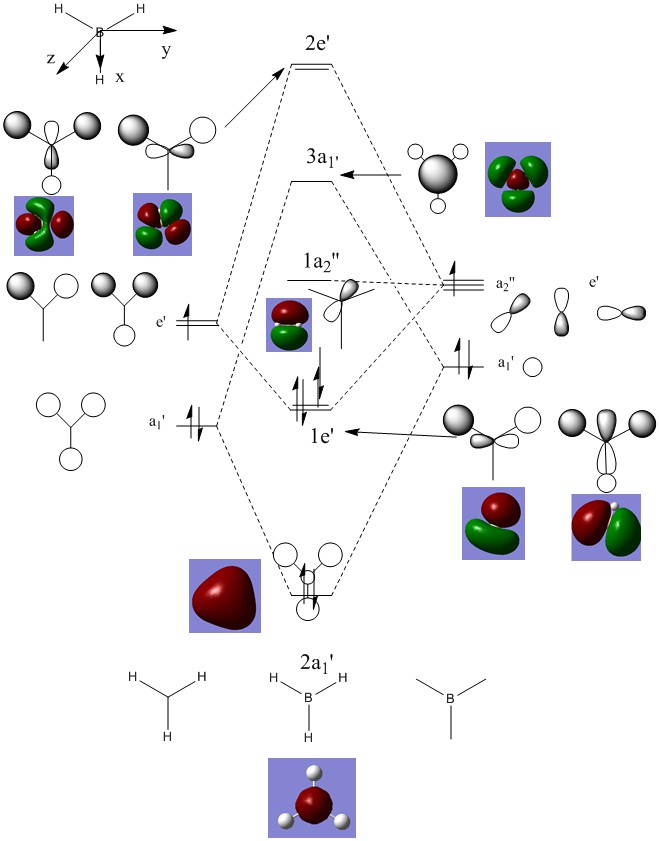
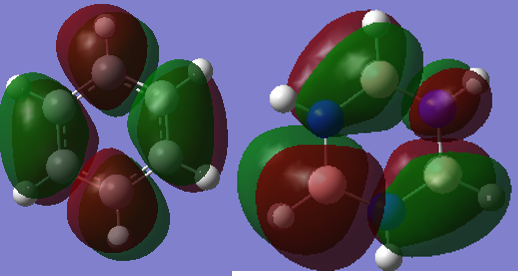
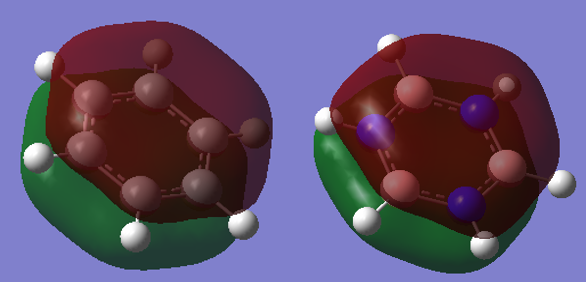
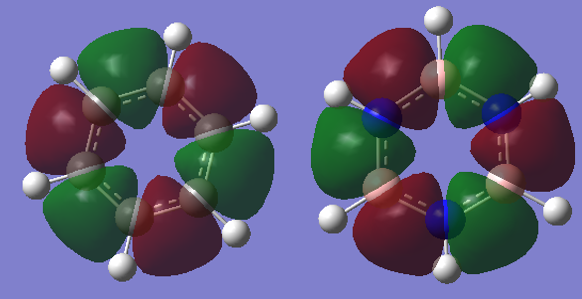
Smf115 (talk) 08:15, 17 May 2018 (BST)Good attempt at the charge analysis with mention of symmetry and electronegativity. Smf115 (talk) 08:15, 17 May 2018 (BST)Nice range of MOs chosen, however, the energies of the MOs can't be compared. Good mention of electronegativity however, the overall character and mention whether they were sigma- or pi- orbitals should have been made for a better comparison.
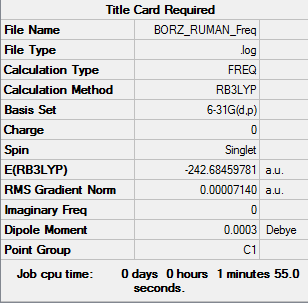
Aromatic molecules have a ring of resonance bonds that provide more stability than similar geometric arrangements of structures containing the same set of atoms. Compounds can be classed as aromatic if they have 4n+2 pi electrons. This is known as Huckle’s rule and therefore can be used to prove that aromatic molecules do not have to be planar. For example, the aromatic compound cyclooctatetraene conforms to a non-planar ‘tub’ conformation, and has 8 pi electrons. Aromaticity is commonly thought to arise from its 6 Pz orbitals which form a pi bond, leading to the formation of a delocalised ring of electron density above and below the ring. However, sigma orbitals can contribute to this delocalisation ring, and in few cases, only some fragments from p orbitals can be thought to contribute to this system.
Smf115 (talk) 08:15, 17 May 2018 (BST)Nice cover of the basic conceptes of aromatiticty. However, the discussion is a bit brief and mention of more complex ideas, such as sigma-aromaticty, and reference to MOs is needed to develop it further.
Smf115 (talk) 08:15, 17 May 2018 (BST)Overall a good attempt at the report and project section.