Rrp17
Molecular Reaction Dynamics
EXERCISE 1: H + H2 system
If H collides with a H2 molecule, there system can be defined as an AB and C. This is a slightly pointless statement as "an AB and C" doesn't really mean anything! Make sure you are always clear and spell everything out very explicitly, i.e. saying the atoms can be labelled... etc.Rk2918 (talk) 13:25, 3 June 2019 (BST)
How is the transition state defined?
The transition state is the maximum on the minimum energy path that links reactants to products; this point is where, ∂V(ri)/∂ri=0, the gradient of the potential energy is zero and the distance ri = rAB = rBC .
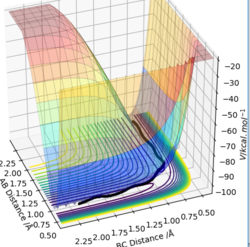
It can be seen that the transition state can be distinguished from a local minimum by plotting a tangent at the inflection point of the minimum energy path. The first derivative, ∂V2/∂q1q12>0and the second derivative ∂V2/∂q2q22<0 This would give you a maximum however, a tangent perpendicular to the qBC would derive the minimum; this location is known as the saddle point and thus the transition state of a reaction. The transition state is a saddle point, it is where the minimum , q1 and max ,q2 intersect.
Yes, great understanding shown here. Also (I can infer you already know this from your answer but it's always good to be detailed so...) on the perpendicular tangent you mention on which the TS is a minimum, the second partial derivate would be positive as it is a minimum. Also make sure you say second partial derivative not just second derivative. Otherwise good. Rk2918 (talk) 13:28, 3 June 2019 (BST)
Transition state position
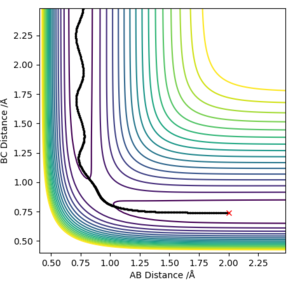
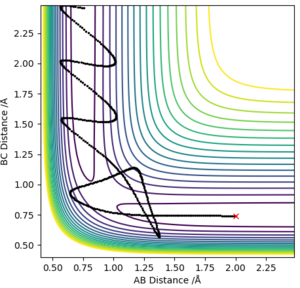
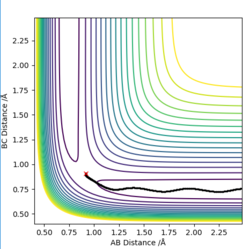
H + H2 is symmetric and hence, the transition state must have r1 = r2, the momentum are zero and there is no gradient at the ridge. The location of the transition state can can be seen in plot 2 and the transition state position (rts) was found to be 0.9077 Å. This was determined by choosing a random value of r1 and changing the inter-nuclear distance until the force equalled zero.
Good, but until what "force" equalled zero? Rk2918 (talk) 13:29, 3 June 2019 (BST)
Minimum energy path and trajectory
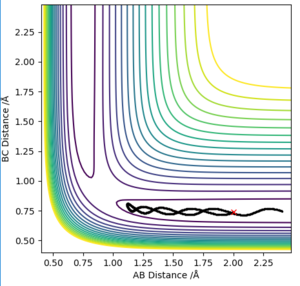
The trajectory is a reaction path of minimum energy where the velocities/momenta are set to zero in each time step. This is where we displayed the transition state position by r1=rts + 0.01 and the momenta is zero. This is shown in plot 3.
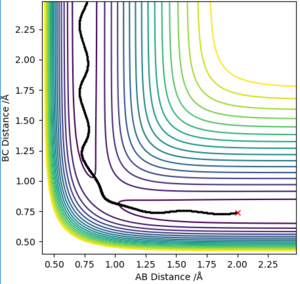
However, MEP cannot characterise a reaction in terms of the motion of atoms. The reaction calculation under 'Dynamics' that is shown in plot 4.
It can be seen that the MEP plot is a plateaued line as the kinetic energy is reset to zero at every interval and, there molecules are not vibrating however, with the dynamic plot, the line is seen as oscillating as the molecules have momentum.
| MEP | Dynamic | |
|---|---|---|
 |
 |
Good use of diagrams to explain and good understanding of the difference in the calculations. Make sure that at the very start of your explanation you are clear about what you are discussing. I.e. here instead of "The trajectory is a reaction path.." etc you should say "A MEP calculation computes the trajectory of minimum energy.." etc especially as you are comparing two methods it is good to be clear of which one we are talking about.Rk2918 (talk) 13:31, 3 June 2019 (BST)
Reactive and nonreactive trajectories
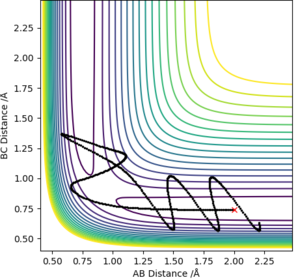
It is clear that the higher the total energy the more likely the reaction will go to completion as the energy is greater than the activation energy. However, it can be seen that with the last two contour plots, the products do not form-another variable affects the outcome of the reaction other than activation energy!
Yep basically the determining factors about whether or not the system will be reactive is not as simple as a threshold energy value above which the reactants will react. Rk2918 (talk) 13:33, 3 June 2019 (BST)
Assumptions of Transition State Theory
The Transition State Theory explains how the rate of a chemical reaction by which the reaction passes through a transition state and it has three assumptions:
-The energy of the particles follow a Boltzmann distribution -The reactants and transition state structure is in equilibrium -The transition state structure does not revert back to the reactants once the reactants form the transition state
Firstly, this should be cited. Secondly, while this is true, it doesn't fully answer the question - how would these assumptions affect the calculated rate of reaction and the comparison of this rate to real-life rates (the accuracy of it)?Rk2918 (talk) 13:33, 3 June 2019 (BST)
Exercise 2: F-H-H system
Using the energy surface, the F + H2 is an exothermic reaction as the products are lower in energy than the reactants.This bond breaking process must mean that the H-F bond is stronger than the H-H and this relates t the ionic nature of the bond due to the large difference in electronegativity between the atoms. Thus, the reverse reaction must be endothermic.
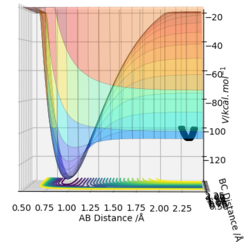
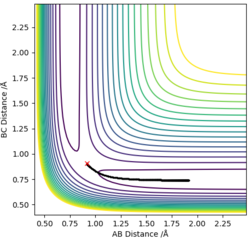
The approximate position of the transition state
Using a MEP contour plot, the saddle point (where there is no momentum) was determined. AB=1.908 Å , BC=0.745 Å. The use of an internuclear distance vs time also highlighted this.
| Contour Plot | Zoom of Contour | |
|---|---|---|
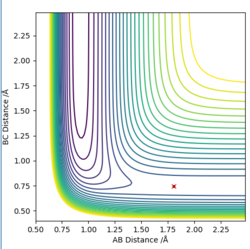 |
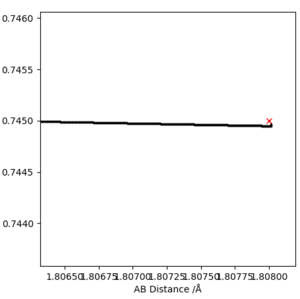 |
Activation energy
The activation energy for both reactions was determined by calculating the difference between the reactants/products and the transition state using an energy vs time plot .
-For the H + HF reaction the activation energy was found to be 30 kcal/mol.
-For the H2 + F reaction the activation energy was found to be 0.026 kcal/mol. This was determined by zooming in on the energy vs time plot: number = 5000 and the BC distance was deviated to 1.82 angstroms and the activation energy is the difference between the total and potential energies.
Mechanism for the release of the reaction energy
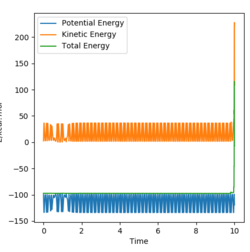
Given that this reaction is exothermic, the thermal energy released would result in an overall increase in kinetic energy and a fall of the potential. Moreover, this exothermic reaction can be determined with calorimetry with sufficient insulation to prevent heat loss.
The initial conditions that result in a reactive trajectory: HH distance of 0.74 HF distance of 2.3 HF momentum of -2.2 HH momentum of -1.9
The results are shown below and show that the potential energy is a mirror of the kinetic energy and the potential energy converts to kinetic- this is how energy is conserved.
Distribution of energy between different modes
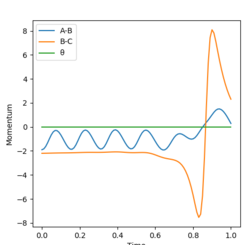
Polanyi's rules1 explain that the efficiency of the reaction is affected by the distribution of energy between different modes; an exothermic reaction has an early transition state and translational energy plays a more fundamental role than vibrational energy in the efficiency of the reaction and vice versa. The rHH momentum corresponds to the vibrational energy and the rHF corresponds to the translational energy.
-For the H2 + F reaction, it can be seen using a dynamic contour plot, when translational energy is greater than vibrational energy, the reaction is efficient and is complete. This exothermic reaction follows Polanyi's rules.
| Incomplete | Complete | |
|---|---|---|
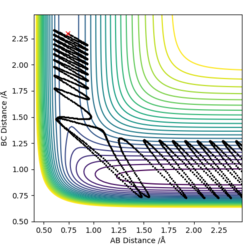 |
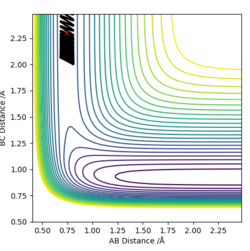 |
-For the HF + F reaction, the transition state is late and vibrational energy, according to Polyani's rules, is more important than translational and allows a more efficient reaction for this endothermic reaction.
| Complete | inComplete | |
|---|---|---|
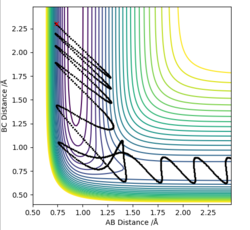 |
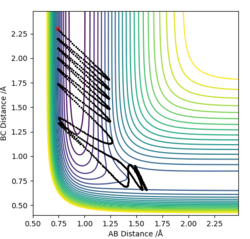 |
Good understanding, explanation and use of examples.Rk2918 (talk) 13:37, 3 June 2019 (BST)
References
[1]Theoretical Study of the Validity of the Polanyi Rules for the Late-Barrier Cl + CHD3 Reaction, Zhaojun Zhang, Yong Zhou,† and Dong H. Zhang, dx.doi.org/10.1021/jz301649w | J. Phys. Chem. Lett. 2012, 3, 3416−3419