Rohirrimlim
Appearance
NH3 Molecule
Summary
Calculation Type = FREQ Calculation Method = RB3LYP Basis Set = 6-31G(d,p) Charge = 0 Spin = Singlet E(RB3LYP) = -56.55776873 a.u. RMS Gradient Norm = 0.00000485 a.u. Imaginary Freq = 0 Dipole Moment = 1.8466 Debye Point Group = C3V
Item Table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES Predicted change in Energy=-5.986283D-10 Optimization completed.
NH3 Molecule |
Bond angle and Bond length
The N-H bond length is 1.01798Å The H-N-H bond angle is 105.741 degree
Literature Values
Literature value for the N-H bond length in NH3 is 101.24pm, H-N-H bond angle is 106.7 degree (CRC Handbook of Chemistry and Physics, 94th ed. http://www.hbcpnetbase.com. Page 9-26.) The value obtained through optimisation is very close to literature value determined through experiments.
Vibration
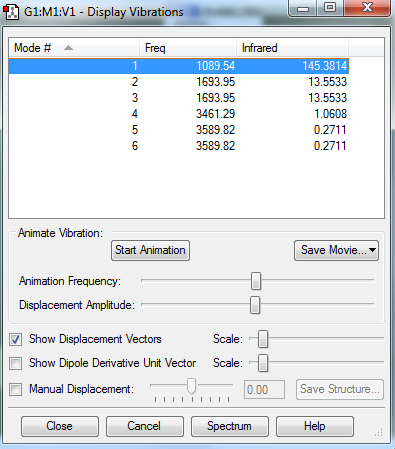
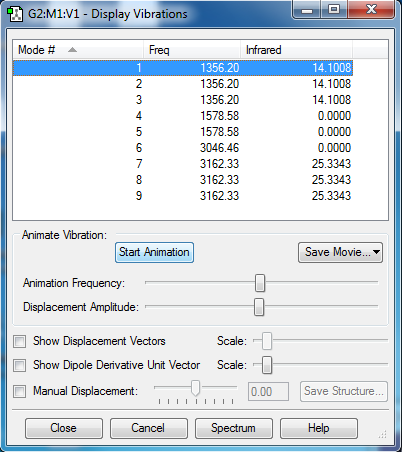
how many modes do you expect from the 3N-6 rule?
Ans: 6
which modes are degenerate (ie have the same energy)?
Ans: Mode 2 and 3 are degenerate as they have the same frequency.Similarly, Mode 5 and 6 are degenerate.
which modes are "bending" vibrations and which are "bond stretch" vibrations?
Ans: Mode 1,2 and 3 are bending vibrations. Mode 4,5 and 6 are stretching vibrations.
which mode is highly symmetric?
Ans: Mode 4
one mode is known as the "umbrella" mode, which one is this?
Ans: Mode 1
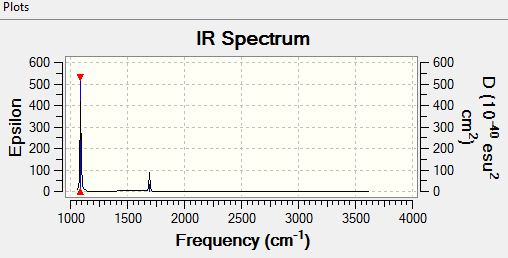
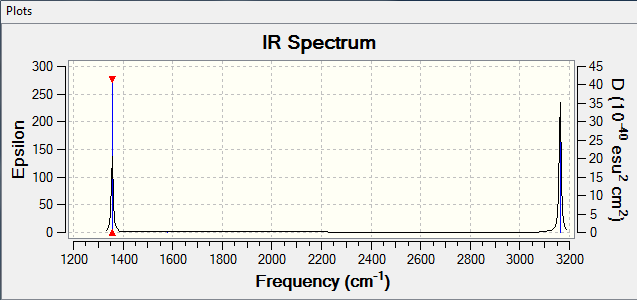
how many bands would you expect to see in an experimental spectrum of gaseous ammonia?
Ans: Mode 1 has an intensity of 145.
Mode 2 and 3 have a combined intensity of 27(as they are degenerate).
Mode 4 has an intensity of 1
Mode 5 and 6 have a combined intensity of 0.54
The intensity of mode 4,5 and 6 are negligible compared to the intensity of Mode 1
Hence 2 bands are expected at 1089.54 cm-1 and 1693.95 cm-1 respectively
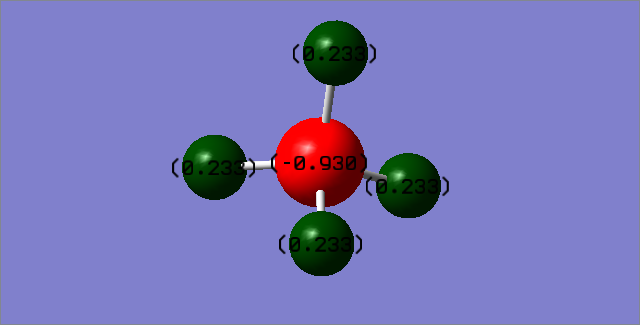
Charge distribution
Nitrogen is expected to carry a negative charge as it is very electronegative and will pull electron density from hydrogen to itself. Hydrogens are expected to carry positive charge as the electron around the hydrogen is pulled away.
N2 Molecule
Summary
Calculation Type = FREQ Calculation Method = RB3LYP Basis Set = 6-31G(d,p) Charge = 0 Spin = Singlet E(RB3LYP) = -109.52359111 a.u. RMS Gradient Norm = 0.02473091 a.u. Imaginary Freq = Dipole Moment = 0.0000 Debye Point Group = D*H Job cpu time: 0 days 0 hours 0 minutes 58.0 seconds.
Item Table
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES Predicted change in Energy=-3.401260D-13 Optimization completed.
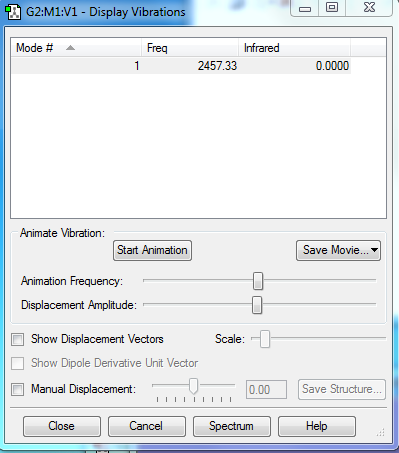
Vibration
As N2 is a diatomic linear molecule, from 3N-5 Rule, only one vibration mode would be expected.
Charge distribution
N2 is consisted of two identical atoms and thus the charge on each atom is expected to be zero.
H2 Molecule
Summary
Calculation Type = FREQ Calculation Method = RB3LYP Basis Set = 6-31G(d,p) Charge = 0 Spin = Singlet E(RB3LYP) = -1.17853936 a.u. RMS Gradient Norm = 0.00000017 a.u. Imaginary Freq = 0 Dipole Moment = 0.0000 Debye Point Group = D*H Job cpu time: 0 days 0 hours 0 minutes 44.0 seconds.
Item Table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES Predicted change in Energy=-1.164080D-13 Optimization completed.
Vibration
As H2 is a diatomic linear molecule, from 3N-5 Rule, only one vibration mode would be expected.
Charge distribution
H2 is consisted of two identical atoms and thus the charge on each atom is expected to be zero.
Reaction Energy of Haber Process
E(NH3)=-56.55776873 a.u
2*E(NH3)=-113.1155375 a.u
E(N2)=-109.52359111 a.u
E(H2)=-1.17853936 a.u
3*E(H2)=-3.53561808 a.u
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=-0.05632831 a.u
=-147.89KJ/mol
The negative enthalpy change indicates an exothermic reaction. Therefore the product is more stable.
The accuracy of calculation is checked by comparing it to the work done by Dingming Zhang, who obtained an enthalpy change of -147.89KJ/mol as well
CH4 Molecule
Summary
Calculation Type = FREQ Calculation Method = RB3LYP Basis Set = 6-31G(d,p) Charge = 0 Spin = Singlet E(RB3LYP) = -40.52401404 a.u. RMS Gradient Norm = 0.00003263 a.u. Imaginary Freq = 0 Dipole Moment = 0.0000 Debye Point Group = TD Job cpu time: 0 days 0 hours 0 minutes 56.0 seconds.
Item Table
Item Value Threshold Converged? Maximum Force 0.000063 0.000450 YES RMS Force 0.000034 0.000300 YES Maximum Displacement 0.000179 0.001800 YES RMS Displacement 0.000095 0.001200 YES Predicted change in Energy=-2.256038D-08 Optimization completed.
CH4 Molecule |
Bond angle and Bond length
The C-H bond length is 1.09197Å The H-C-H bond angle is 109.471 degree
Literature Values
Literature value for the N-H bond length in NH3 is 1.17Å , H-N-H bond angle is 109.5 degree
("Methane". National Institute of Standards and technology.)
The value obtained through optimisation is very close to literature value determined through experiments.
The value of bond angle is almost identical as the shape of the molecule is effectively tetrahedral.
There is a slight deviation in bond length which is within acceptable range
Vibration
As CH4 molecule is non-linear, from 3N-6 Rule, 9 different vibration modes are expected. Mode 1,2 and 3 are degenerate as they have the same energy. Similarly, Mode 4 and 5 are degnerate, Mode 7 and 8 and 9 are degenerate. Mode 1 2 and 3(degenerate) have a combined intensity of 42 Mode 7 8 and 9(degenerate) have a combined intensity of 76 Mode 4 and 5 , Mode 6 do not show an intensity Therefore, two bands would be expected
Charge Distribution
As Carbon is more electronegative than hydrogen, it is expected to carry a negative charge as it pulls electron density from Hydrogen to itself. Likewise, Hydrogen would be carrying a positive charge as the electron density around it is drawn towards to Carbon.
Molecular orbitals
Overview
Individual molecular orbitals are listed below in the order of increasing energy
MO 1
Energy=-10.16707 au This is the molecular orbital for the 1s electrons of the carbon atom. It is non-bonding and therefore very deep in energy.
MO 2
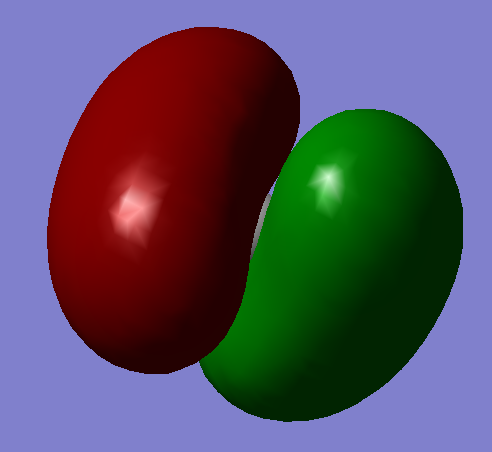
Energy=-0.69041 au This bonding molecular orbital is formed through the mixing of the 2s orbital on carbon and the 1s orbital on hydrogen.
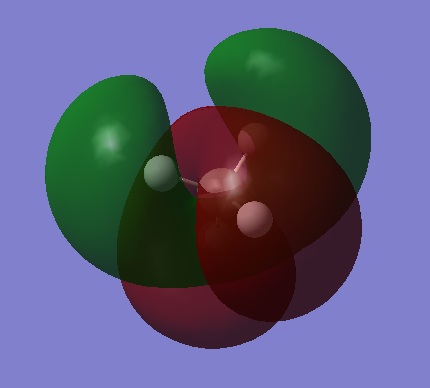
MO 3,4&5
Energy=-0.38831 au These three bonding molecular orbitals are degenerate, all having the same energy. They are formed through the mixing of the 2p orbitals on carbon and 1s orbitals on hydrogen. The 2p orbital is higher in energy than 2s so these MOs are higher in energy than MO2 They are the HOMO
MO 6
Energy=0.11824 au This is the LUMO and the anti-bonding orbital of MO2 (2s-1s mixing)
MO 7,8&9
Energy=0.17677 au These three MOs are the anti-bonding orbitals of MO3,4&5.