Rlk3917 inorganic computational
BH3
B3LYP 6-31Gd/p
BH3 frequency and MOs File Name = RKW-BH3-FREQ File Type = .log Calculation Type = FREQ Calculation Method = RB3LYP Basis Set = 6-31G(d,p) Charge = 0 Spin = Singlet E(RB3LYP) = -26.61532348 a.u. RMS Gradient Norm = 0.00007899 a.u. Imaginary Freq = 0 Dipole Moment = 0.0000 Debye Point Group = D3H Job cpu time: 0 days 0 hours 0 minutes 36.0 seconds.
Item Value Threshold Converged? Maximum Force 0.000158 0.000450 YES RMS Force 0.000079 0.000300 YES Maximum Displacement 0.000622 0.001800 YES RMS Displacement 0.000311 0.001200 YES
Link to log file
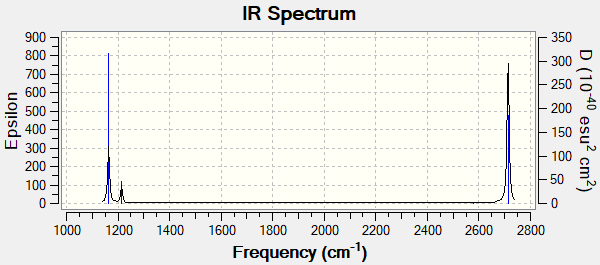
Low frequencies --- -0.2456 -0.1129 -0.0054 44.0270 45.1846 45.1853 Low frequencies --- 1163.6049 1213.5924 1213.5951
BH3 |
Vibrations
| # | Frequency / cm-1 | Intensity |
|---|---|---|
| 1 | 1164 | 92 |
| 2 | 1214 | 14 |
| 3 | 1214 | 14 |
| 4 | 2580 | 0 |
| 5 | 2713 | 126 |
| 6 | 2713 | 126 |
The 4th vibration is IR inactive, as this corresponds to the symmetrical stretch of all three B-H bonds.
Ng611 (talk) 17:38, 8 May 2019 (BST) Symmetry labels and assignments (i.e.: type of mode, out-of-plane bend, in-plane bend, etc.) are missing. Also, why are there only three peaks in the IR spectrum, but 6 modes in the table?
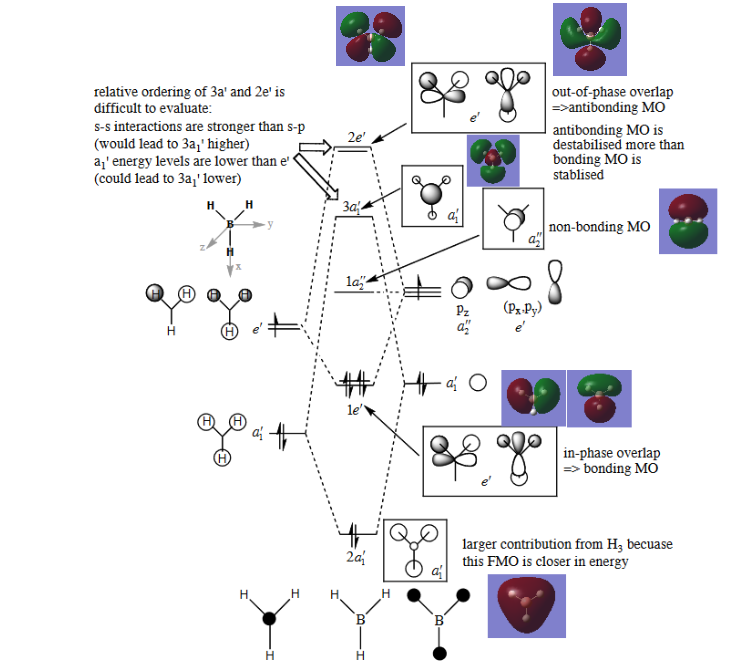
MOs
Molecular Orbital diagram from: P. Hunt's tutorial notes, http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf, (accessed May 2019)
The calculated molecular orbitals do not deviate significantly from those derived through a qualitative approach, showing that it is a powerful tool for the approximate determination of molecular orbitals.
Ng611 (talk) 17:43, 8 May 2019 (BST) Note that the 3a1 and 2e' MOs have do deviate somewhat from qualitative MO theory. Try to think about how they deviate.
NH3 & NH3BH3
NH3
B3LYP 6-31Gd/p
NH3 File Name = NH3 File Type = .log Calculation Type = FREQ Calculation Method = RB3LYP Basis Set = 6-31G(d,p) Charge = 0 Spin = Singlet E(RB3LYP) = -56.55776873 a.u. RMS Gradient Norm = 0.00000323 a.u. Imaginary Freq = 0 Dipole Moment = 1.8465 Debye Point Group = C3V Job cpu time: 0 days 0 hours 0 minutes 57.0 seconds.
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000014 0.001800 YES RMS Displacement 0.000009 0.001200 YES
Link to log file
Low frequencies --- -0.0127 -0.0022 0.0006 7.1034 8.1048 8.1051 Low frequencies --- 1089.3834 1693.9368 1693.9368
NH3 |
NH3BH3
B3LYP 6-31Gd/p
NH3BH3 File Name = NH3BH3 File Type = .log Calculation Type = FREQ Calculation Method = RB3LYP Basis Set = 6-31G(d,p) Charge = 0 Spin = Singlet E(RB3LYP) = -83.22468892 a.u. RMS Gradient Norm = 0.00005935 a.u. Imaginary Freq = 0 Dipole Moment = 5.5651 Debye Point Group = C3V Job cpu time: 0 days 0 hours 0 minutes 28.0 seconds.
Item Value Threshold Converged? Maximum Force 0.000121 0.000450 YES RMS Force 0.000057 0.000300 YES Maximum Displacement 0.000570 0.001800 YES RMS Displacement 0.000318 0.001200 YES
Link to log file
Low frequencies --- -0.0253 -0.0031 0.0004 17.0476 17.0501 36.9367 Low frequencies --- 265.7521 632.2129 639.3374
NH3BH3 |
Relative Energy
E(BH3)=-26.61532 a.u. E(NH3)=-56.55777 a.u. E(NH3BH3)=-83.22469 a.u.
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]=-83.22469-(-26.61532-56.55777) =-0.05160 a.u =-135 kJmol-1
This is a weak bond, compared with a C-C bond in the analagous ethane which is ~360 kJmol-1.
Ng611 (talk) 17:49, 8 May 2019 (BST) You need to add a reference here for the literature value you cited.
NI3
B3LYP/6-31G(d,p)LANL2DZ
Link to log file
NI3 optimisation File Name = NI3-OPTIMISATION File Type = .log Calculation Type = FREQ Calculation Method = RB3LYP Basis Set = Gen Charge = 0 Spin = Singlet E(RB3LYP) = -88.80858845 a.u. RMS Gradient Norm = 0.00004431 a.u. Imaginary Freq = Dipole Moment = 1.3092 Debye Point Group = C3V Job cpu time: 0 days 0 hours 0 minutes 27.0 seconds.
Item Value Threshold Converged? Maximum Force 0.000102 0.000450 YES RMS Force 0.000075 0.000300 YES Maximum Displacement 0.000858 0.001800 YES RMS Displacement 0.000629 0.001200 YES
Low frequencies --- -12.3845 -12.3781 -5.6129 -0.0040 0.0194 0.0711 Low frequencies --- 100.9307 100.9314 147.2333
NH3BH3 |
N-I bond length = 2.184 Å
Project
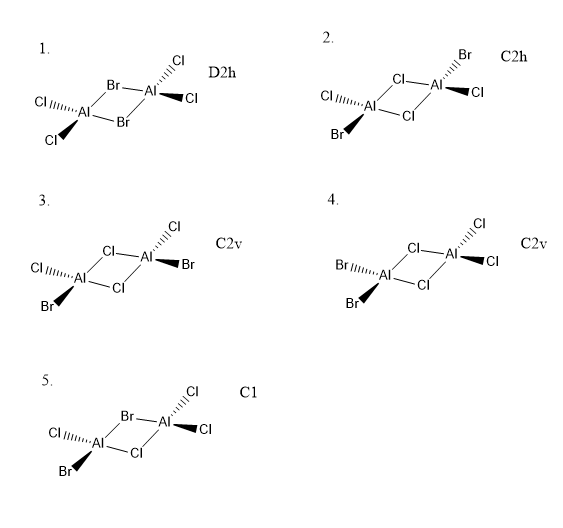
Isomer 1
Method:B3LYP/ Al 6-31G(d,p); Cl, Br LANL2DZ
Link to log file
Al2_1 File Name = AL2_1 File Type = .log Calculation Type = FREQ Calculation Method = RB3LYP Basis Set = Gen Charge = 0 Spin = Singlet E(RB3LYP) = -571.43283094 a.u. RMS Gradient Norm = 0.00002738 a.u. Imaginary Freq = 0 Dipole Moment = 0.0000 Debye Point Group = D2H Job cpu time: 0 days 0 hours 0 minutes 21.0 seconds.
Item Value Threshold Converged? Maximum Force 0.000034 0.000450 YES RMS Force 0.000015 0.000300 YES Maximum Displacement 0.000402 0.001800 YES RMS Displacement 0.000113 0.001200 YES
Low frequencies --- -2.5130 -1.5345 -0.0024 -0.0020 -0.0019 1.9565 Low frequencies --- 16.6689 62.3371 84.7970
Al2Br2Cl4 Isomer 1 |
Isomer 2
Method:B3LYP/ Al 6-31G(d,p); Cl, Br LANL2DZ
Link to log file
Al2_2 File Name = Al2_2_1 File Type = .log Calculation Type = FREQ Calculation Method = RB3LYP Basis Set = Gen Charge = 0 Spin = Singlet E(RB3LYP) = -571.43792379 a.u. RMS Gradient Norm = 0.00013242 a.u. Imaginary Freq = 0 Dipole Moment = 0.0276 Debye Point Group = C1 Job cpu time: 0 days 0 hours 0 minutes 37.0 seconds.
Item Value Threshold Converged? Maximum Force 0.000318 0.000450 YES RMS Force 0.000076 0.000300 YES Maximum Displacement 0.000554 0.001800 YES RMS Displacement 0.000243 0.001200 YES
Low frequencies --- -3.7118 -2.2032 -0.0006 0.0009 0.0015 2.2873 Low frequencies --- 18.7371 47.6135 71.2551
Al2Br2Cl4 Isomer 2 |
Relative Energy
-571.43792379 a.u.--571.43283094 a.u. = -0.00509 a.u. = -13.4 kJmol-1
Isomer 2 is lower in energy. This is because the large bromine atoms are not the bridging species, and are not forced into close proximity causing steric clashes.
AlBrCl2
Method:B3LYP/ Al 6-31G(d,p); Cl, Br LANL2DZ
Link to log file
Al File Name = Al File Type = .log Calculation Type = FREQ Calculation Method = RB3LYP Basis Set = Gen Charge = 0 Spin = Singlet E(RB3LYP) = -285.70300759 a.u. RMS Gradient Norm = 0.00005532 a.u. Imaginary Freq = 0 Dipole Moment = 0.3536 Debye Point Group = CS Job cpu time: 0 days 0 hours 0 minutes 23.0 seconds.
Item Value Threshold Converged? Maximum Force 0.000104 0.000450 YES RMS Force 0.000063 0.000300 YES Maximum Displacement 0.000729 0.001800 YES RMS Displacement 0.000610 0.001200 YES
Low frequencies --- -0.0024 -0.0024 -0.0020 1.8269 2.9309 4.9652 Low frequencies --- 119.7790 132.7679 182.5577
AlBrCl2 |
Dissociation Energy
ΔE = E(Al2Br2Cl4) - 2 x E(AlBrCl2) = -571.43792379 a.u. -2 x -285.70300759 a.u. = 0.03191 a.u. = -83.8 kJmol-1
The monomer is more stable than the dimer.
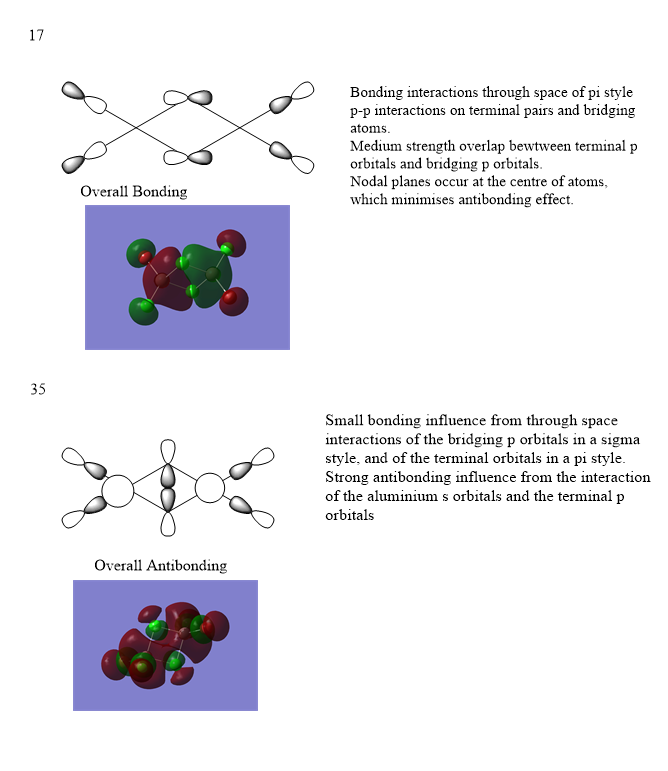
Ng611 (talk) 18:07, 8 May 2019 (BST) 3x MOs were required.
Ng611 (talk) 18:07, 8 May 2019 (BST) The LCAO decomposition for your first MO is incorrect; the terminal chlorine/bromine atoms should interact via their s orbitals.
Ng611 (talk) 18:11, 8 May 2019 (BST) Second MO decomposition is good, well done.