Riccardo wiki
NH3 Analysis
NH3 Summary
| Calculation Method | Basis Set | Final Energy | RMS Gradient | Point Group |
|---|---|---|---|---|
| RB3LYP | 6-31G(d,p) | -56.55776873 au | 0.00000485 | C3v |
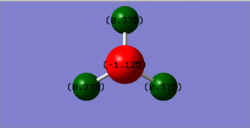
Optimised bond length= 1.01798
Bond angle= 105.7
Item table
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986279D-10
Optimization completed.
-- Stationary point found.
Jmol NH3
test molecule |
This Molecule is the most stable for ammonia
NH3 Vibrations
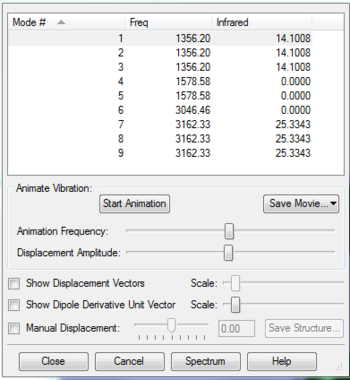
| Number of modes | Degenerate modes | Modes that bend | Modes that stretch | Which is symmetric |
|---|---|---|---|---|
| 6 | 5 and 6, 2 and 3 | 1,2 and 3 | 4,5 and 6 | 1 and 4 |
Umbrella mode= 1
expected bands in an experimental spectrum of gaseous ammonia?= 2
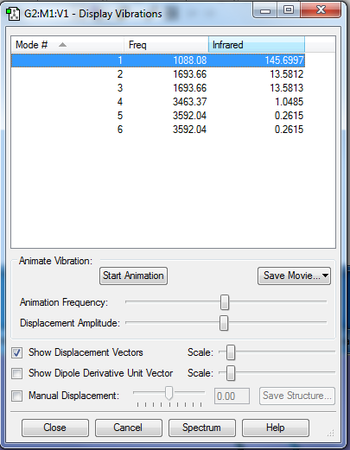
NH3 Charge Distribution
Nitrogen (red)= -1.25 Hydrogen (green)= 0.375
N2
Summary
| Calculation Method | Basis Set | Final Energy | RMS Gradient | Point Group |
|---|---|---|---|---|
| RB3LYP | 6-31G(d,p) | -109.52412868 au | 0.00000060 | D*H |
Optmised bond length= 1.1055 A
Bond angle= 180
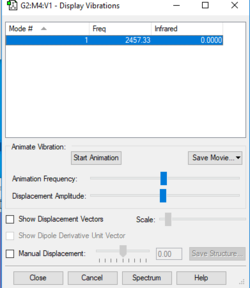
Frequency= 2457.33, IR= 0
Item table
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.401116D-13
Optimization completed.
-- Stationary point found.
test molecule |
N2 Vibrations
N2 Charge Distribution
Diatomic with same atoms of equal negativity, hence no charges
H2
H2 Summary
| Calculation Method | Basis Set | Final Energy | RMS Gradient | Point Group |
|---|---|---|---|---|
| RB3LYP | 6-31G(d,p) | -1.17853936 au | 0.00000017 | D*H |
Optimised bond length 0.74279 A
Bond angle=180
Item table
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
Jmol H2
test molecule |
Vibrations H2
Frequency= 4464.68 , IR=0
H2 Charge Distribution

Diatomic with same atoms of equal negativity, hence no charges
Haber-Bosch reaction
N2 + 3H2 -> 2NH3
| E(NH3) | 2*E(NH3) | E(N2) | E(H2) | 3*E(H2) |
|---|---|---|---|---|
| -56.55776873 au | -113.115537 au | -109.52412868 au | -1.17853936 au | -3.53561808 au |
Change in energy= 2*E(NH3)-[E(N2)+3*E(H2)]=- 0.005579024 au = -146.47727512 KJ/mol
reactants are less stable
CH4
Summary
| Calculation Method | Basis Set | Final Energy | RMS Gradient | Point Group |
|---|---|---|---|---|
| RB3LYP | 6-31G(d,p) | -40.52401404 au | 0.00003263 | TD |
Optimised bond length= 1.09197 A
Bond angle= 109.47
Item table
Item Value Threshold Converged?
Maximum Force 0.000063 0.000450 YES
RMS Force 0.000034 0.000300 YES
Maximum Displacement 0.000179 0.001800 YES
RMS Displacement 0.000095 0.001200 YES
Predicted change in Energy=-2.256043D-08
Optimization completed.
-- Stationary point found.
Jmol CH4
test molecule |
This is the most stable form of methane
Vibrations
| Number of modes | Degenerate modes | Modes that bend | Modes that stretch | Which is symmetric |
|---|---|---|---|---|
| 0 | 1,2 and 3;4 and 5; 7,8 and 9) | 1,2,3 and 4 | 5,6,7,8 and 9 | 6,5 and 4 |
expected bands in an experimental spectrum?= 2
Charge Distribution
CH4 Bending
CH4 MO's
MO 1
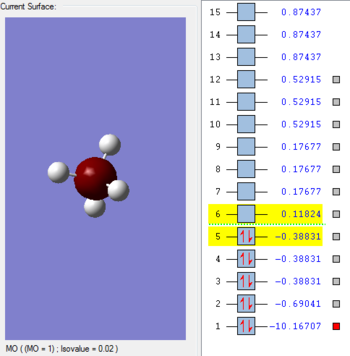
This occupied orbital is very deep in energy with -10.17 au. This 1s from the carbon, which does not contribute towards bonding, makes up the MO. Its small size and stability is due to its proximity to the carbon nucleus and large attractive forces. Very little overlap is seen with the Hydrogens.
MO 2
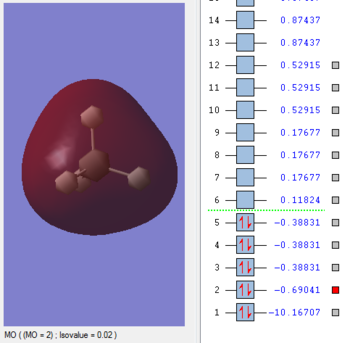
The contribution from each AO is from each atom, to form the MO. Contribution of 2s from Carbon and 1s from each hydrogen atom. There is a large degree of mixing. Quite deep in energy and more than MO 3, however, there is also contribution from the 2p Carbon orbitals. This is a bonding orbital. Its energy is found as -0.69041 au.
MO 3- sp3
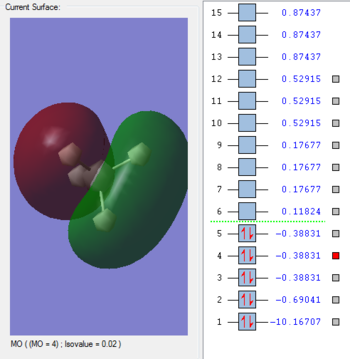
This is the bonding HOMO, given that MO 3,4 and 5 are all degenerate. Three AO P orbitals of Carbon; px, py, pz hybridise with its 2s to bond to to each 1s Hydrogen orbital.Its energy is found to be -0.38831 auand is higher than MO 2 due to the energy of the orbitals involved- being further away from the nucleus.
MO 4-LUMO
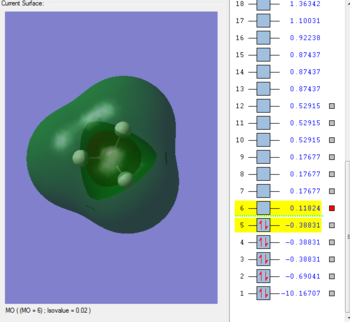
This orbital is unoccupied and the lowest-unoccupied molecular orbital- it is anti-bonding 1s orbital from Carbon. Its energy is positive as 0.11824 au, and is lower in energy than the anti-bonding hybridised MO.
MO 5
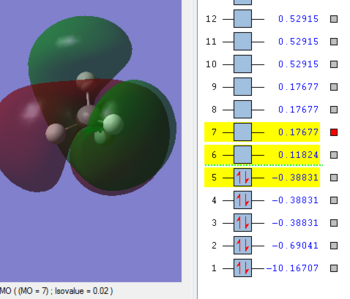
This anti-bonding orbital related to the anti bonding of the hybridised Carbon sp3 and the 1s on each Hydrogen. Higher in energy than the LUMO. Its energy is 0.17677 au and has of course, 2 other MO's that are degenerate to it- MO 8 and 9.
Water
Summary
| Calculation Method | Basis Set | Final Energy | RMS Gradient | Point Group |
|---|---|---|---|---|
| RB3LYP | 6-31G(d,p) | -76.41973740 au | 0.00006276 | C2V |
Optimised bond length= 0.96522
Bond angle= 103.7
Item Table
Item Value Threshold Converged?
Maximum Force 0.000099 0.000450 YES RMS Force 0.000081 0.000300 YES Maximum Displacement 0.000115 0.001800 YES RMS Displacement 0.000120 0.001200 YES Predicted change in Energy=-1.939669D-08 Optimization completed. -- Stationary point found.
Jmol H2O
test molecule |
This Jmol shows the most stable form of the molecule
H2O Vibrations
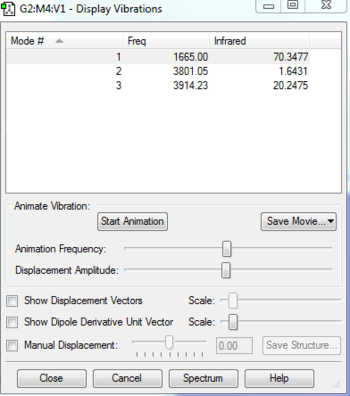
| Number of modes | Degenerate modes | Modes that bend | Modes that stretch | Which is symmetric |
|---|---|---|---|---|
| 3 | 0 | 0 | 3 | 1 and 2 |