Rep:YNX19950213
Investigation of Transition States and Reactivity
Introduction
The development of computational chemistry and computational programs have provided great tools for study chemical reaction's mechanisms. One namely the study of transition states[1], which would be impossible to monitor or simulate with conventional lab experiments. This page is delicate to present some of the most well-known reactions' transition state. And discus the relationships between molecular symmetries and reactivity. Investigate how does Endo and Exo pathway in Diels-Alder reaction differ in energy profile. Which reaction pathway is more favorable in thermodynamic aspect and kinetic aspect. Is there any potential secondary orbital interactions happened during the reactions? If do, is it true for both Endo and Exo pathway? And finally how does Cheletropic reaction differ from Diels-Alder reaction in every aspect.
Nf710 (talk) 18:47, 20 January 2017 (UTC) You should have defined a TS here
Reaction of Butadiene and Ethylene
Reaction Scheme
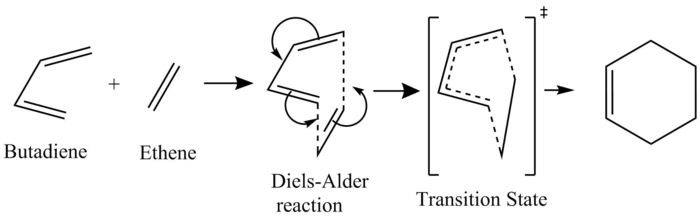
This is a very typical and simple Diels-Alder Reaction[2] between Butadiene and Ethene, illustrated as the MO diagram below.
Molecular Orbitals
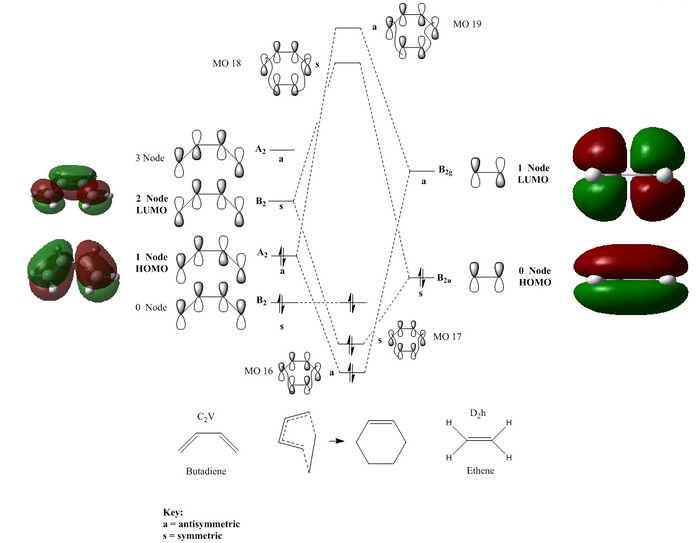
In order to have a successful reaction, symmetries of the HOMO and the LUMO orbitals must match, otherwise the reaction is forbidden. Orbitals can only be either symmetric or anti-symmetric, which can be determined by calculation of their wave functions. As the MO diagram illustrated, the frontier orbitals of butadiene (The HOMO) and ethene (The LUMO) are both symmetric (denoted as s), thus the interaction is allowed. And the interactions between an Anti-symmetric (a) and a Symmetric (s) orbital is forbidden due to their different orbital symmetry.
| MO 16 | MO 17 | MO 18 | MO 19 |
|---|---|---|---|
|
|
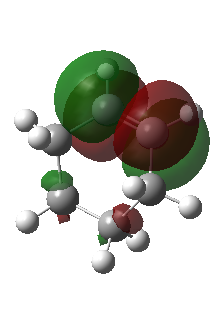
|
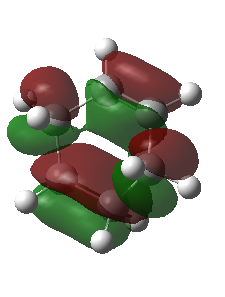
|
| Transition state MO16 | Transition state MO17 | Transition state MO18 | Transition state MO19 |
|---|---|---|---|
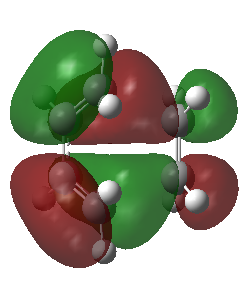
|
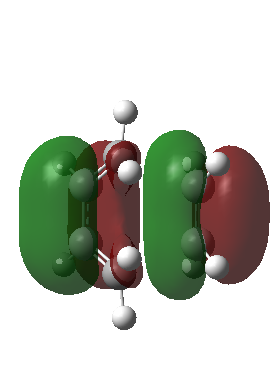
|
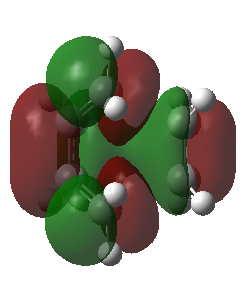
|
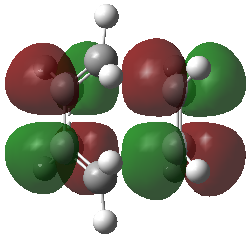
|
The corresponding MO lobes were generated by GaussView 5.0.
For your MO did the MO of the product look like this when you checked in the MOS section?
Reaction analysis: Symmetry and Reactivity
It can be clearly seen that the LUMO of Butadiene interacted with the HOMO of Ethene. In order to have a successful reaction, symmetries of the HOMO and the LUMO orbitals must match, otherwise the reaction is forbidden. Orbitals can only be either symmetric or anti-symmetric, which can be determined by calculation of their wave functions. As the MO diagram illustrated, the frontier orbitals of butadiene (The HOMO) and ethene (The LUMO) are both symmetric (denoted as s), thus the interaction is allowed. And the interactions between an Anti-symmetric (a) and a Symmetric (s) orbital is forbidden due to their different orbital symmetry.
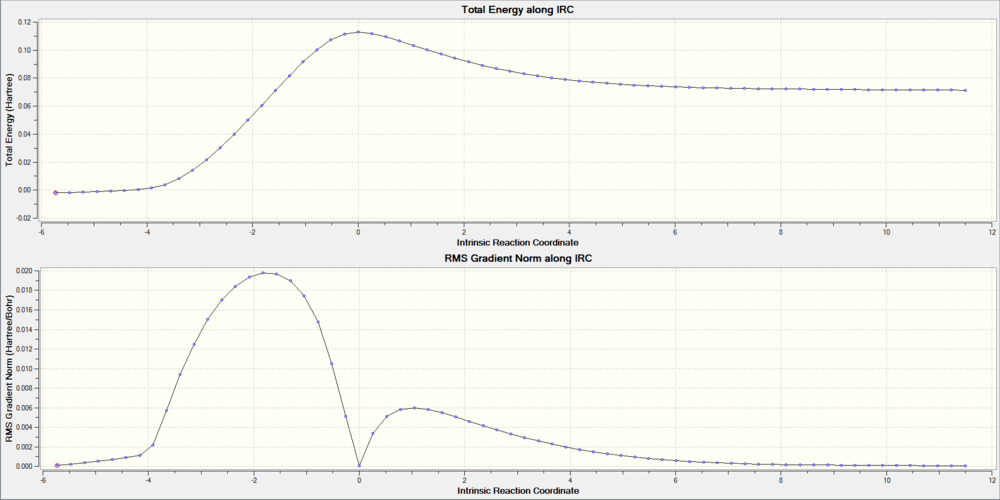
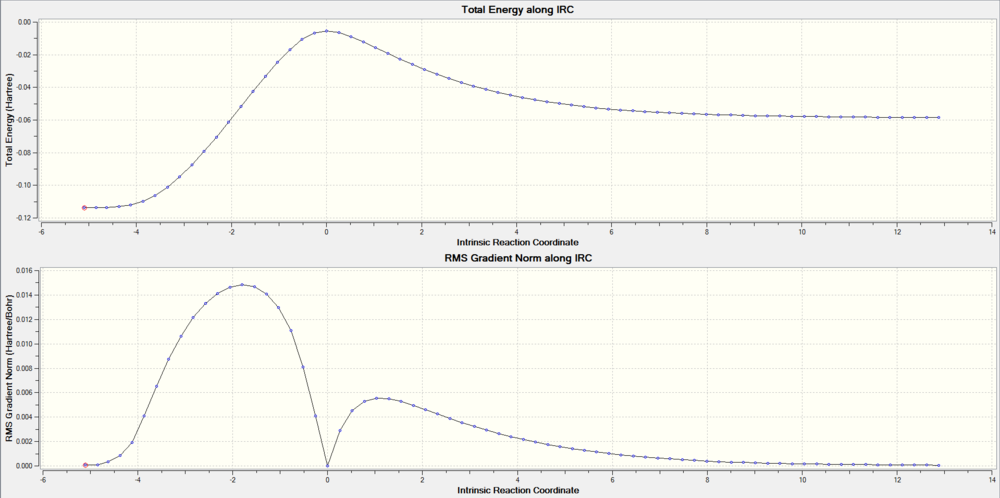
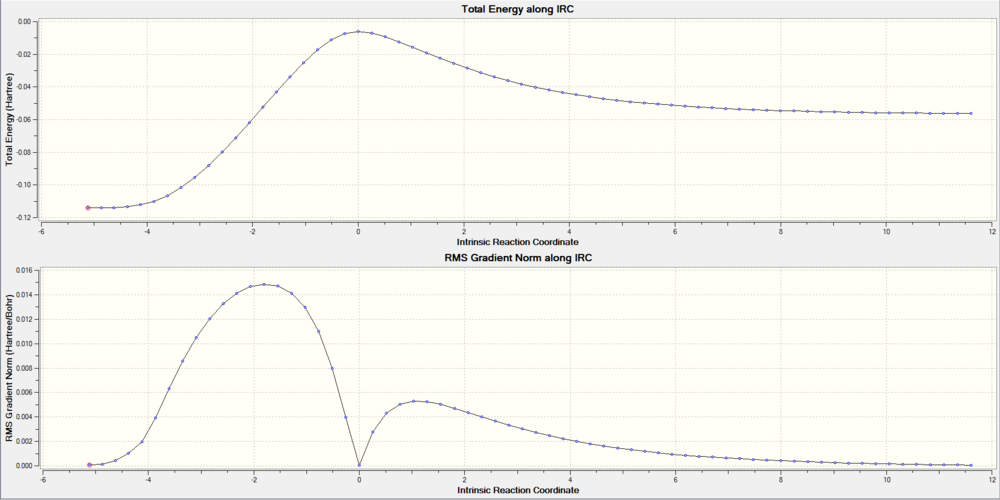
With the help of Intrinsic Reaction Coordinate calculation, we can trace the minimum energy pathway between the reactant and the product on the potential energy surface. The highest point of total energy corresponds to the Transition state.
Visualization of the Transition State
With the help of computationol programs, we can simulate the molecular interactions during the transition state. By looking at the Imaginary Vibrations' of the TS we can have a straight forward idea of how Butadiene and Ethene were react to give the Cyclohexene .
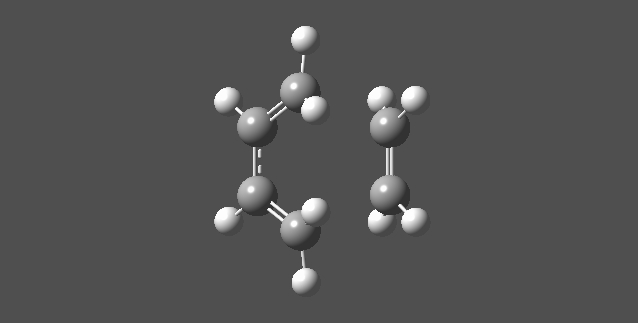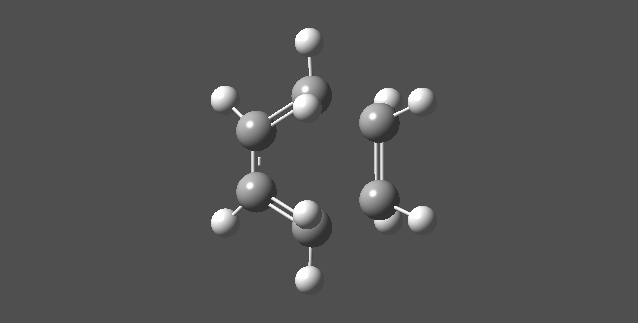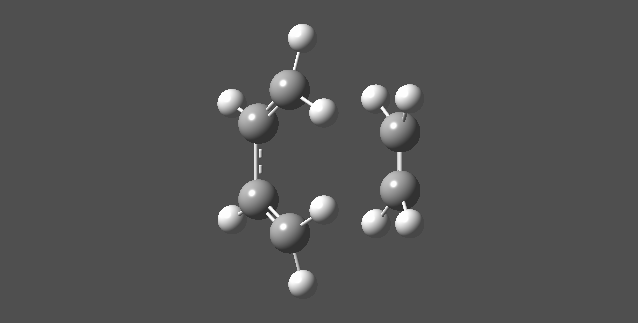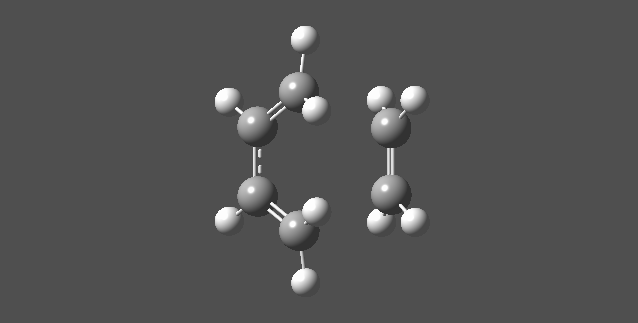
Nf710 (talk) 19:00, 20 January 2017 (UTC) You have drawn very nice MOS etc but you have missed out alot of what was asked in the script, for example you havent measured any bond lengths
Reaction of Cyclohexadiene and 1,3-Dioxole
Reaction Scheme
The reaction have two different pathway, namely Endo and Exo. Which are shown below
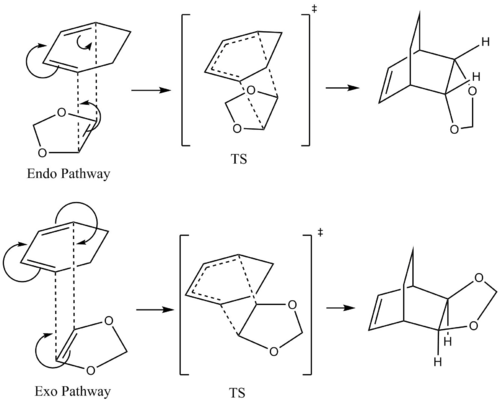
Molecular Orbitals
This is an inverse demand DA reaction because there are two new chemical bonds and a six-membered ring are formed and is between an electron-rich dienophile, namely cyclohexadiene, and an electron-poor diene, namely dioxole. The Dioxole is electron-poor due to the two electron withdrawing Oxygen atom in adjacent to the double bond.
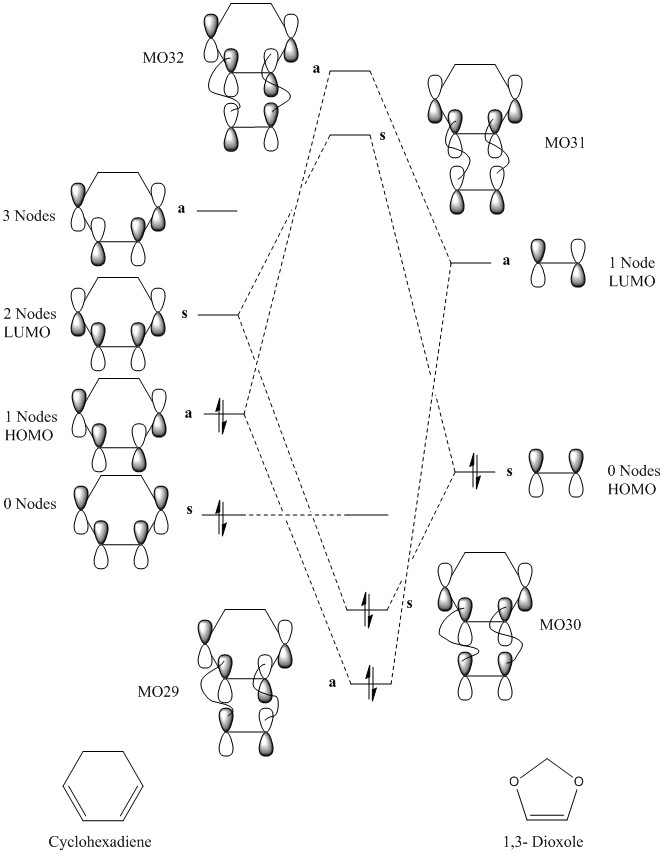
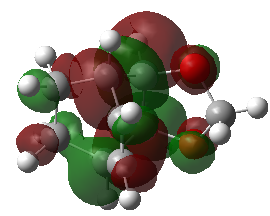
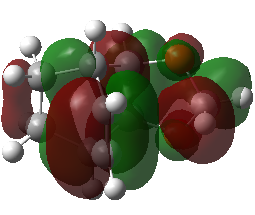
(This MO diagram suggests that the lowest energy MO of cyclohexadiene that you've shown is higher in energy in the TS than the bonding orbitals, and becomes unoccupied. This is not the case, as the orbital is very low down in energy and it remains occupied. You can confirm this by looking at the occupied orbitals in GaussView. Also remember, the number of orbitals and electrons going into an MO diagram must remain the same Tam10 (talk) 14:54, 4 January 2017 (UTC))
| MO 29 | MO 30 | MO 31 | MO 32 |
|---|---|---|---|
|
|

|
|
| Endo Transition State MO29 | Endo Transition State MO30 | Endo Transition State MO31 | Endo Transition State MO32 |
|---|---|---|---|
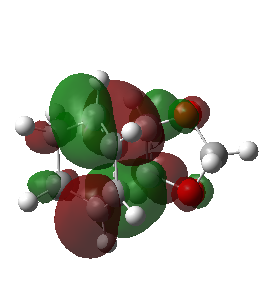
|
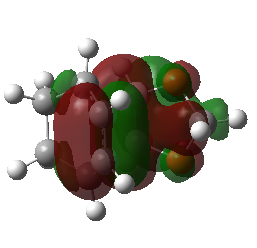
|
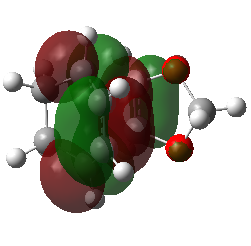
|
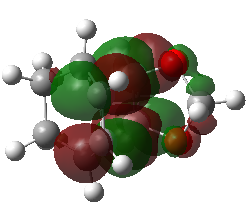
|
| Exo Transition State MO29 | Exo Transition State MO30 | Exo Transition State MO31 | Exo Transition State MO32 |
|---|---|---|---|
|
|
|

|
(These look like PM6 orbitals, which don't show secondary orbital interactions in this case. Also quite hard to see the interactions Tam10 (talk) 14:54, 4 January 2017 (UTC))
Nf710 (talk) 19:06, 20 January 2017 (UTC) Your energies look incorrect I assume they are PM6 energies.
Reaction analysis: Energies
| The energies of the Reaction Profile | Endo(KJ/mol) | Exo(KJ/mol) |
|---|---|---|
| Reactant | -1511893.671 | -1511877.126 |
| Transition State | -1511963.779 | -1511970.525 |
| Product | -1511921.323 | -1511892.187 |
| Ea | 70.108 | 93.399 |
| ΔE | -27.652 | -15.061 |
The Endo pathway having both a more stable product (a bigger ΔE) and a smaller activation energy (Ea), which means that the Endo pathway is both thermodynamically and knetically favoured.
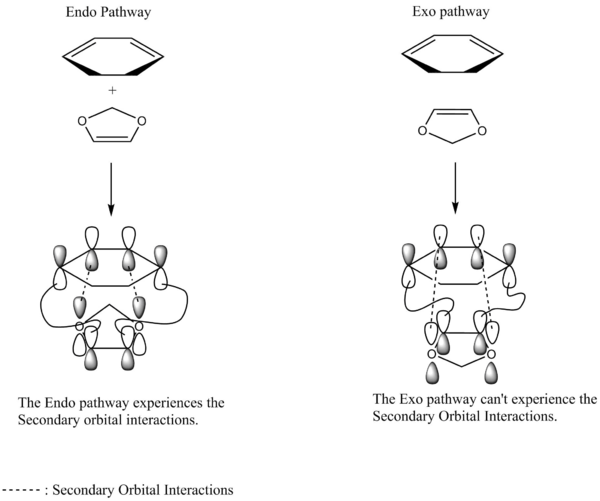
Secondary orbital interaction
(Don't forget to change the phases when flipping parts of your diagram! Tam10 (talk) 14:54, 4 January 2017 (UTC))
The Secondary Orbital Interactions happends between the two oxygen lone pairs and the π* orbital of the diene. The Endo pathway experienced the Secondary Orbital Interactions, however,the Exo reaction pathway can't experience the Secondary Orbital Interactions however, due to the fact that the distance between the two oxygen lone pairs and the π* orbital is too far for the interactions to take place.
Nf710 (talk) 19:10, 20 January 2017 (UTC) Your have clearly shown some understanding of the reaction but you have missed a few key and made a few errors. your SOO is good but you messed up your orbitals. You have also shown no understanding if it is normal or inverse demand.
Diels-Alder vs Cheletropic
Reaction analysis: Reaction profile
| Endo(KJ/mol) | Exo(KJ/mol) | Cheletropic(KJ/mol) | |
|---|---|---|---|
| Reactant | 176.33 | 178.25 | 188.56 |
| Transition State | 241.75 | 237.76 | 260.07 |
| Product | 56.3 | 57.0 | 0.0005 |
| Ea | 65.42 | 59.51 | 71.51 |
| ΔE | -120.03 | -121.25 | -188.55 |
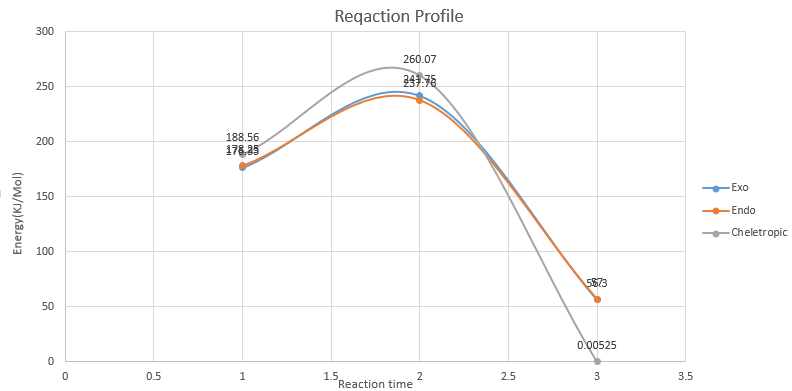
(Don't draw smooth lines like this - it's very misleading as you only have 3 points. If you are to use curved lines, the gradients become important and must be 0 for the stationary points. Better to draw straight lines between the points. Additionally, normalise the reactants to 0 to make comparison easier Tam10 (talk) 14:54, 4 January 2017 (UTC))
From the Reaction Profile and the data, it can be conclude that the Endo pathway is the most Kinetically favoured as it has the lowest activation energy (Ea), thus having the fastest reaction rate. The Cheletropic pathway on the other hand, although having the highest activation energy, but has the biggest ΔE and lowest product energy. The product's free energy is almost zero, which will be extremely stable (irreversible) and thus will be the thermodynamically favourable pathway.
Below are IRC of each reaction pathway, the energy was in Hatree.(1 Hatree = 2625.5 KJ/mol)
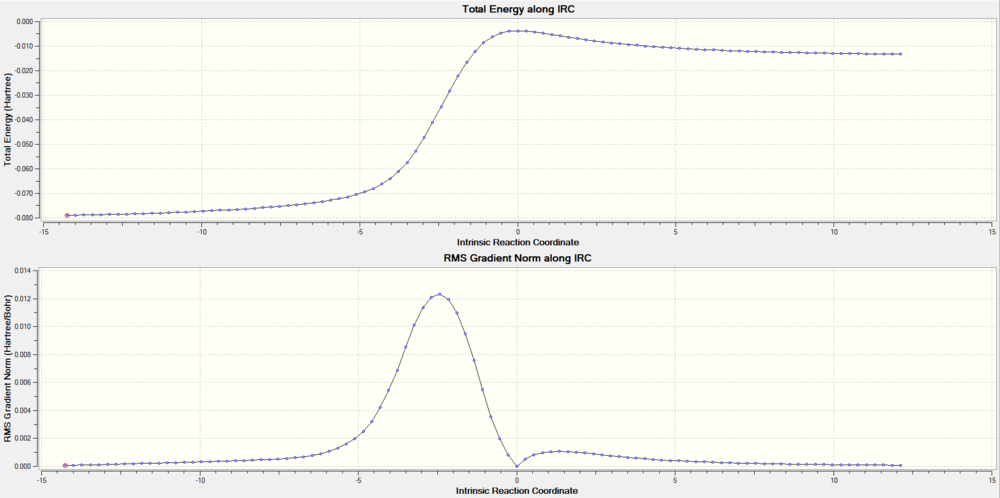

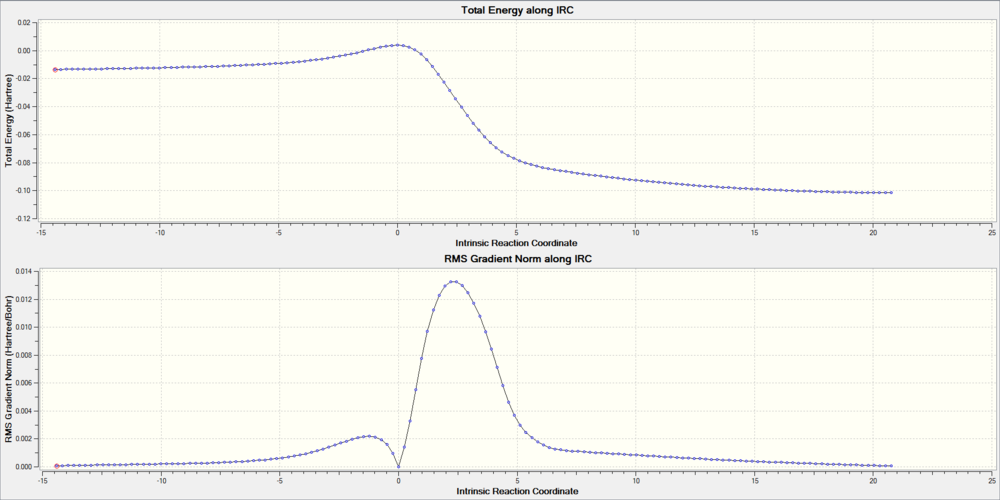
(Not enough text. You've missed out the question about xylylene's instability Tam10 (talk) 14:54, 4 January 2017 (UTC))
Conclusion
With all the studies of the three Ring forming reactions using GaussView, one can simply realized that the reaction energies are really important and useful for confirming hypothetical transition state. And being able to visualize reaction process via animation and generate actual MO lobes are also extremely helpful in the study of reaction mechanism and molecular symmetries.
With the help of computational portal, more accurate 6-31G calculation can be utilized, however, in practice the PM6 was much more commonly used thanks to it's fast processing. The most useful and unique feature of computational chemistry is that it costs nothing, and you have the result almost immediately, one can thus quickly get feedback and redesign his/her experiment.
