Rep:Xlt15 FURTHER
Further Work: Electrocyclic Reaction
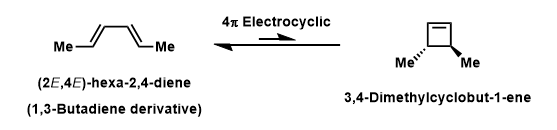
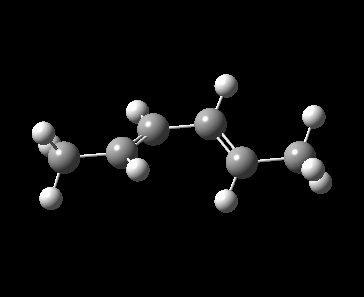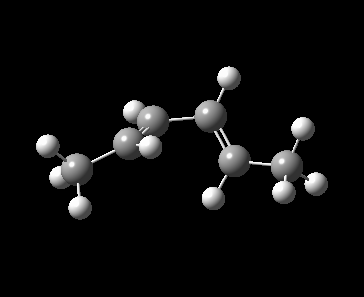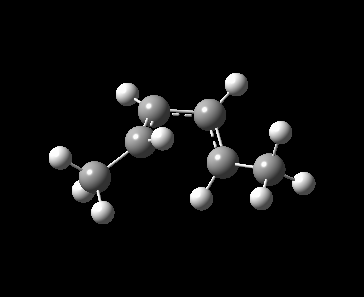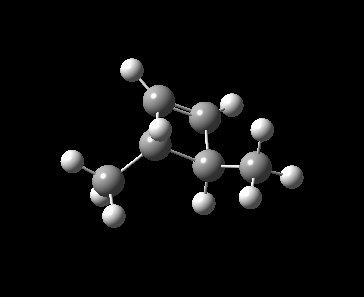
A 4π electrocyclic reaction in Figure 1 is investigated as a further work to the computational lab. An electrocyclic reaction is the formation or the breaking of σ bonds across the end of a conjugated π system [1]. The equilibrium favoured towards left hand side because of the ring strain in the 4-membered ring.
Method Used In Optimization and Analysis
Method 3 was employed in locating the transition state by which the product, cyclobut-1-ene derivative was drawn and optimized to minimum at PM6 level. With the optimized product, the C-C single bond formed during the pericyclic reaction was deleted, froze at 2.20 Å and optimized to minimum at PM6 level to identify the frozen guess transition state. The guess TS structure was then optimized at PM6 level and the PM6 optimized TS was used to run a IRC calculation. For reactant, 1,3-butadiene derivative, it was obtained from the first frame of IRC calculation and optimized to minimum at PM6 level.
Optimized Reactant, Transition State, and Product at PM6 Level
| Table 1: Optimized Reactants, Transition State and Product at PM6 Level. | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1,3-Butadiene Derivative | Transition State | 3,4-Dimethylcyclobut-1-ene | ||||||
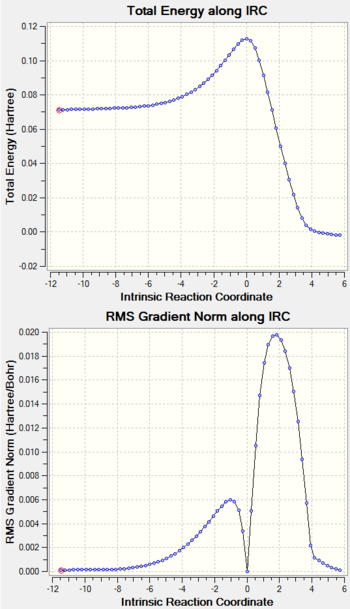
The geometry of reactants, transition structure and product are checked to properly converge with their respective stationary points found in log files. In addition, transition structure has only one imaginary frequency and that imaginary frequency is then visualized to ensure a correct transition state structure is obtained. There is no imaginary frequency obtained in reactants and products.
IRC Calculation
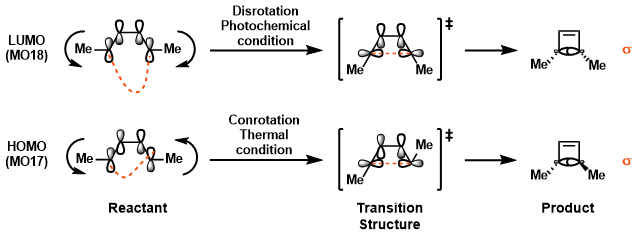
Conrotation or Disrotation Analysis Using MO
| Table 4. MO of Reactant, Transition State and Product. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Molecular Orbital | 1,3 Butadiene Derivative | Transition State | 3,4-Dimethylcyclobut-1-ene | ||||||
| MO18
LUMO |
|||||||||
| MO17
HOMO |
|||||||||
| Table 5. Orbital Correlation Diagram of Reactant, TS and Product. | |
|---|---|
| Orbital Correlation Diagram | Explanation |
 |
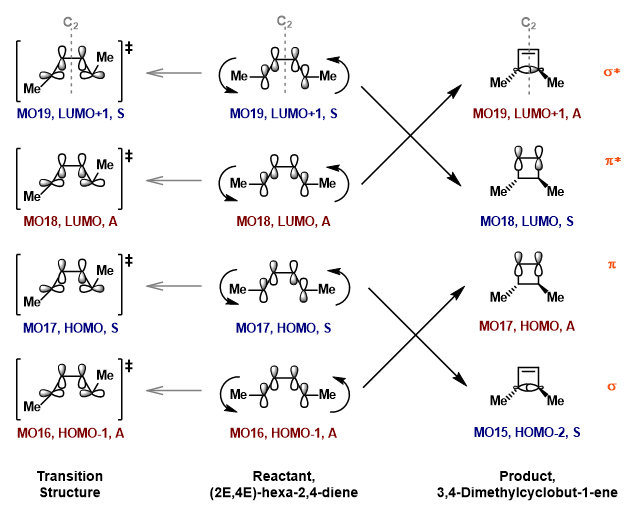
The orbital correlation diagram in Figure 2 shows the simplified version of the MOs observed in reactant, transition state structure and product, with their actual HOMO and LUMO displayed in Table 4. The MOs are labelled symmetric (S) or antisymmetric (A) using the persistent symmetry element, C2-rotational axis. The type of bonding or antibonding interaction in product is also shown and coloured in orange.
|
(Very good work with the correlation diagram. However your labelling of the symmetries is a bit off. It is unexpected but a particular MO may switch between symmetric and antisymmetric along the reaction path as it passes the Mobius transition state Tam10 (talk) 10:35, 26 February 2018 (UTC))
Woodward-Hoffmann Rule For Electrocyclic Reaction [1].
| Table 4. Orbital Symmetry Allowed Reaction by Woodward-Hoffmann Rule. | ||
|---|---|---|
| Number of π Electon | Thermal Condition | Photochemical Condition |
| 4n | Conrotatory | Disrotatory |
| Antarafacial | Suprafacial | |
| 4n+2 | Disrotatory | Conrotatory |
| Suprafacial | Antarafacial | |
Log File For IRC Calculation
IRC Calculation of PM6 Optimized TS: File:XLT15SUETS IRC.LOG
Exercise 1
https://wiki.ch.ic.ac.uk/wiki/index.php?title=Xlt15_TS
Exercise 2
https://wiki.ch.ic.ac.uk/wiki/index.php?title=Xlt15_Ex2
Exercise 3
https://wiki.ch.ic.ac.uk/wiki/index.php?title=Xlt15_Ex3
References in Further Work
- ↑ 1.0 1.1 J. Clayden, N. Greeves, S. Warren, P. Wothers, Organic Chemistry, Oxford University Press Inc., New York, 2001. Cite error: Invalid
<ref>tag; name ":0" defined multiple times with different content - ↑ E. V. Anslyn, D. A. Dougherty, Modern Physical Organic Chemistry, University Science Books, Sausalito, United States, 2006.
- ↑ R. Hoffmann, R. B. Woodward, Acc. Chem. Res, 1968, 1(1), 17-22, DOI:10.1021/ar50001a003
- ↑ 4.0 4.1 4.2 4.3 B. Dinda, Essentials of Pericyclic and Photochemical Reactions, Springer International Publishing, Switzerland, 2017.