Rep:Xlt15 Ex3
Exercise 3: Diels-Alder vs Cheletropic
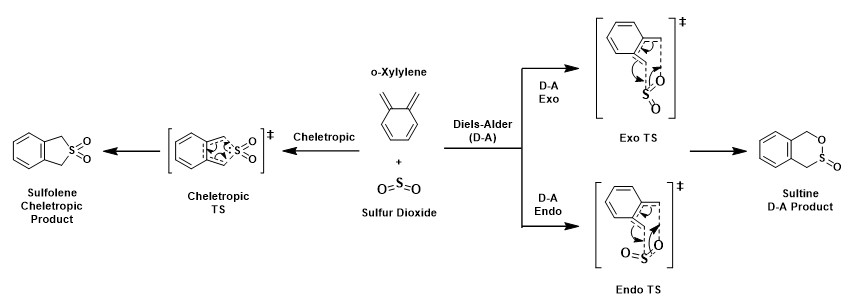
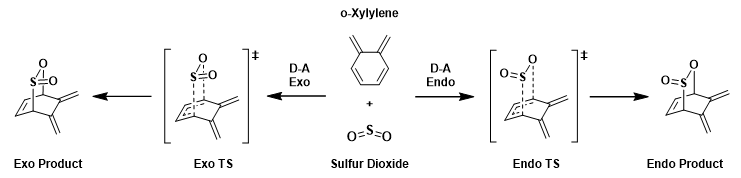
The cycloaddition of o-xylylene and sulfur dioxide can proceed in 2 ways, [4+2]-cycloaddition (D-A exo and D-A endo pathway) and [4+1]-cheletropic cycloaddition. Cheletropic reaction is a subclass of cycloaddition involving a concerted and synchronous formation or breaking of two new σ bonds to a single atom [1] [2]. Both D-A and cheleteropic reaction involve 6π electrons. In cheletropic reaction (giving sulfolene), the S atom contributes its lone pair of electron to the 6π pericyclic transition state whereas in the hetero-D-A reaction, the o-xylylene reacts reversibly with SO2 suprafacially, producing sulfite as product [3]. It is worthnoting that there is an increase in the coordination number on S from 4 to 6 in cheletropic reaction whereas the S coordination number remains the same in D-A reaction.
Method Used In Optimization and Analysis
Method 3 is employed in locating the transition state by which the product is drawn and optimized to minimum at PM6 level. With the optimized product, the C-S and C-O single bonds formed during the reaction are deleted and these bonds are froze at 2.40 Å and 2.00 Å respectively. It is then optimized to minimum at PM6 level to identify the frozen guess transition state. The guess TS structure is optimized at PM6 level and the PM6 optimized TS was used to run a IRC calculation. For reactants, o-xylylene and sulfur dioxide, they are each obtained from first frame of IRC calculation and optimized to minimum at PM6 level.
For the reaction between second cis-butadiene fragemnt in o-xylylene and sulfur dioxide, method 2 was employed whereby a guess transition state structure was drawn and the C-S and C-O distances were froze at 2.40 Å and 2.00 Å respectively. This guess TS is then optimized at PM6 level and used to obtain IRC calculation. The product obtained from the last frame in the IRC calculation was optimized to minimum at PM6 level.
Optimized Reactants, Transition Structure and Products at B3LYP/6-31G(d) level
| Table 1: Optimized Reactants at PM6 level | |||||
|---|---|---|---|---|---|
| o-Xylylene | SO2 | ||||
| Table 2: Optimized Transition State and Product at PM6 level | ||||||
|---|---|---|---|---|---|---|
| Types of Reaction and Pathway | Transition State | Product | ||||
| Diels-Alder Exo | ||||||
| Diels-Alder Endo | ||||||
| Cheletropic Reaction | ||||||
The geometry of reactants, transition state and product are checked to properly converge with their respective stationary points found in log files. In addition, the transition state has only one imaginary frequency and it is then visualized to ensure a correct transition state structure is obtained. There is no imaginary frequency obtained in reactants and products.
IRC Calculation
(This isn't a stepwise reaction unless there are intermediates along the reaction path. All you can say here is the bonds are formed at different time - you can't even really say which one is "first" as Gaussview is just using length cutoffs to determine when it should draw a line between the atoms Tam10 (talk) 10:06, 26 February 2018 (UTC))
Thermochemistry
| Table 4: Thermochemistry Data at PM6 Level. | |
|---|---|
| Species | Sum of Electronic and Thermal Free Energies/ kJmol-1 |
| o-Xylylene | +468.1083 |
| SO2 | -313.1408 |
| Sum of Reactant Energy | +154.9675 |
| Diels-Alder Exo TS | +241.7456 |
| Diels-Alder Endo TS | +237.7627 |
| Cheletropic TS | +260.0847 |
| Diels-Alder Exo Product | +56.3301 |
| Diels-Alder Endo Product | +56.9839 |
| Cheletropic Product | +0.0131 |
| Table 5: Reaction Barriers and Reaction Energies. | ||
|---|---|---|
| Reaction Pathway | Reaction Barriers/ kJmol-1 | Reaction Energies/ kJmol-1 |
| Diels-Alder Exo | +86.78 | -98.64 |
| Diels-Alder Endo | +82.80 | -97.98 |
| Cheletropic Reaction | +105.12 | -154.95 |
Reaction Profile
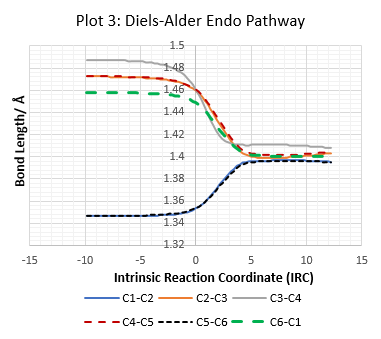
Change in Bonding of o-Xylylene during The Reaction
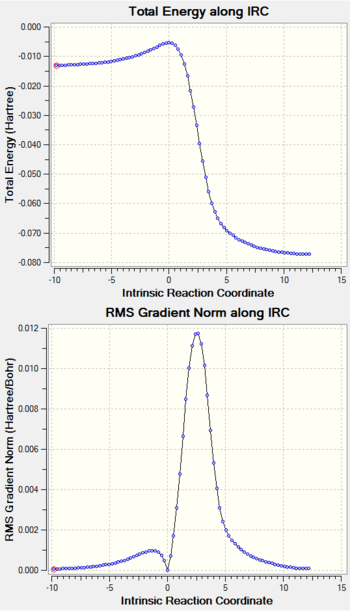
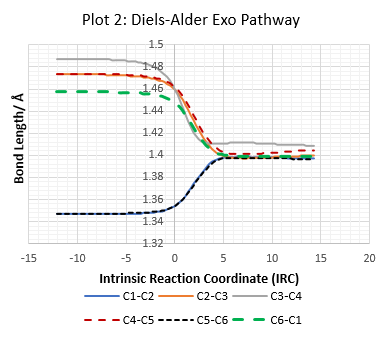
| Table 7: IRC Output For D-A Exo, D-A Endo and Cheletropic Reactions. | ||
|---|---|---|
| Bond Length against IRC | ||
 |
 |
 |
|
All C-C bond lengths against IRC are plotted using the numbering system in Figure 2.
| ||
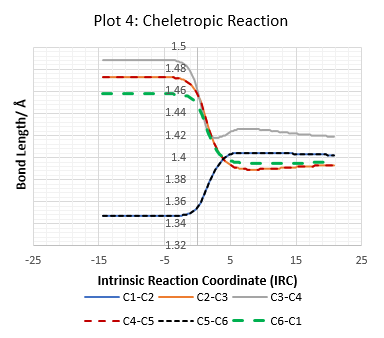
Reaction of Second Cis-Butadiene in Diels-Alder Reaction
As there is a second 1,3-butadiene in s-cis conformation, it can also undergo Diels-Alder (D-A) reaction with the sulfur dioxide.
PM6 Level Optimized Transition Structure and Product
| Table 8: Optimized Transition State and Product at PM6 Level. | ||||||
|---|---|---|---|---|---|---|
| Types of Reaction and Pathway | Transition State | Product | ||||
| Diels-Alder Exo | ||||||
| Diels-Alder Endo | ||||||
The geometry of reactants, transition structure and product are checked to properly converge with their respective stationary points found in log files. In addition, transition structure has only one imaginary frequency and that imaginary frequency is then visualized to ensure a correct transition state structure is obtained. There is no imaginary frequency obtained in reactants and products.
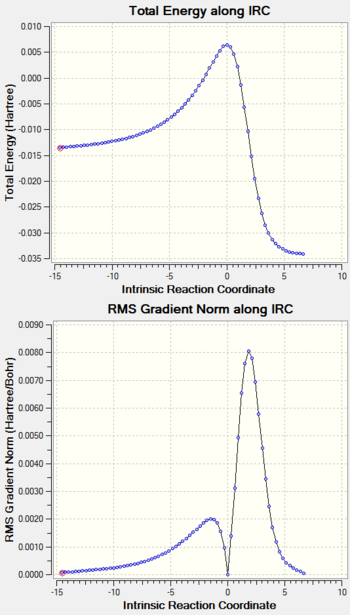
IRC Calculation
| Table 9: IRC Calculation of PM6 Optimized Transition Structures. | ||
|---|---|---|
| IRC Output | Reaction Pathways | |
| Diels-Alder Exo | Diels-Alder Endo | |
| Reaction Progress | 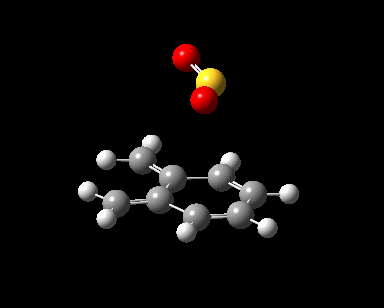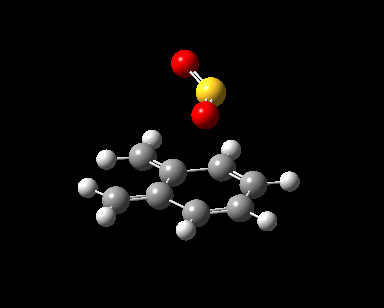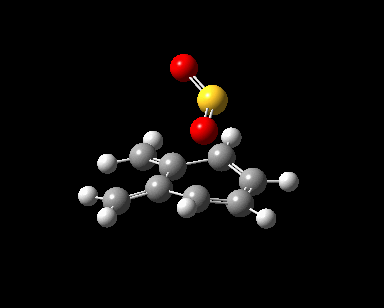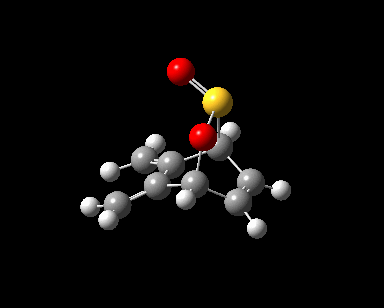 |
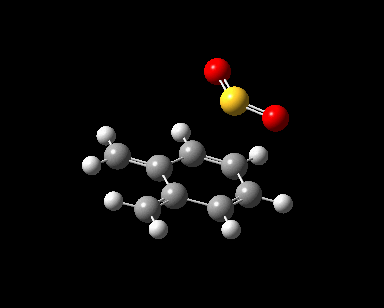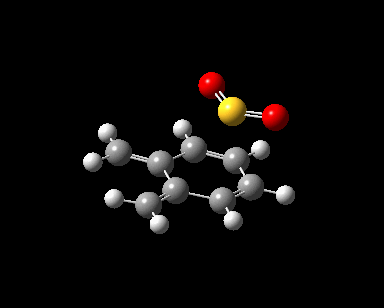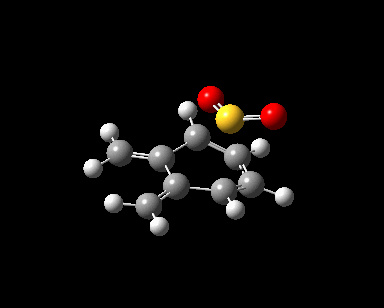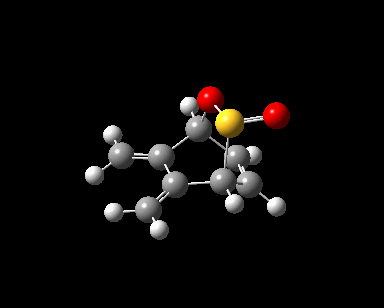 |
| IRC Calculation |  |
 |
Thermochemistry
| Table 10: Thermochemistry Data at PM6 Level. | |
|---|---|
| Species | Sum of Electronic and Thermal Free Energies/ kJmol-1 |
| Sum of Reactant Energy | +156.2041 |
| Diels-Alder Exo TS | +275.8245 |
| Diels-Alder Endo TS | +267.9848 |
| Diels-Alder Exo Product | +176.7067 |
| Diels-Alder Endo Product | +172.2591 |
| Table 11: Reaction Barriers and Reaction Energies | ||
|---|---|---|
| Reaction Pathway | Reaction Barriers/ kJmol-1 | Reaction Energies/ kJmol-1 |
| Diels-Alder Exo | +119.62 | +20.50 |
| Diels-Alder Endo | +111.78 | +16.05 |
By comparing Table 5 and 11, the D-A reaction of second cis-butadiene and SO2 has a higher activation barrier to reach TS, by about 33 kJmol-1 for exo pathway and by roughly 29 kJmol-1 for endo pathway. In addition, both D-A exo and endo pathway of second cis-butadiene and SO2 is endothermic, meaning that these reactions require an energy input. The exo and endo products from the second-cis butadiene are rather destabilized and have higher Gibbs free energy compared to the sum of reactant energy, hence the formation of product is not spontaneous. Also, they are higher in energy than the previous D-A reaction due to the lack of aromaticity in product. Thus, the D-A reaction between the second cis-butadiene and SO2 is concluded to be very thermodynamically and kinetically unfavourable.
(Very detailed section and good work. For the conclusions it's probably best to keep them in their own sections to avoid confusion Tam10 (talk) 10:06, 26 February 2018 (UTC))
Log File for IRC Calculation of PM6 Optimized Transition Structures
Diels-Alder Exo TS: File:XLT15EXOTS IRC3.LOG
Diels-Alder Endo TS: File:XLT15ENDOTS IRC3.LOG
Cheletropic reaction TS: File:XLT15CHELETS IRC3.LOG
Diels-Alder Exo TS of second cis-butadiene: File:XLT152BUTAEXOTS IRC.LOG
Diels-Alder Exo TS of second cis-butadiene: File:XLT15ENDO2BUTATS IRC.LOG
Exercise 1
https://wiki.ch.ic.ac.uk/wiki/index.php?title=Xlt15_TS
Exercise 2
https://wiki.ch.ic.ac.uk/wiki/index.php?title=Xlt15_Ex2
Further Work
https://wiki.ch.ic.ac.uk/wiki/index.php?title=Xlt15_FURTHER
Conclusion
Both PM6 and B3LYP/6-31G(d) methods were successfully used to optimize the reactants, transition states and products. The transition state was identified by the presence of a single imaginary frequency and there is no such negative frequency in a properly optimized reactant and product. IRC calculation was then performed on the PM6 optimized transition state.
The Diels-Alder (D-A) reaction between 1,3-butadiene and ethylene is a concerted and spontaneous C-C bond formation. This is illustrated by the imaginary frequency of the transition state and the identical C-C separation of the reacting termini. The separation between the reacting termini is less than the Van der Waals distance of 2 C atoms, implying a partially formed bond. Also, only orbitals of the identical symmetry can combine to give a non-zero orbital overlap integral.
The D-A reaction between a cyclohexadiene and 1,3-dioxole is an inverse electon demand reaction, determining by a single point energy calculation to obtain the relative energy levels of the frontier molecular orbital (FMO). Based on Gibbs free energy obtained from a B3LYP/6-31G(d) calculation, the endo-adduct of D-A reaction is more kinetically and thermodynamically favoured than exo-adduct because of the favourable secondary orbital interaction and less steric hindrance in endo product.
The cycloaddition of o-xylylene and sulfur dioxide has D-A exo, D-A endo and cheletropic pathways. Based on their reaction profile, the cheletropic reaction is the most thermodynamically favoured, having the most exothermic reaction energy whereas the D-A endo product is the most kinetically favourable, having the smallest activation barrier to reach TS. All three reaction is highly exothermic due to the gain in aromaticity in product. Based on the thermochemistry data from the PM6 level calculation, the D-A exo and endo reaction of second cis-butadiene and sulfur dioxide are endothermic and require more activation barrier to reach TS, indicating that both are thermodynamically and kinetically unfavourable reaction.
For the electrocyclic reaction, MOs of reactant, TS and product was employed to determine whether it is conrotation or disrotation. It is observed that there is a C2 axis of symmetry preserved during the reaction and hence it is used in symmetry labelling of the MOs. An thermal electrocyclic reaction with (4n)π reaction involve conrotation of the group on the terminal C via a Mobius aromatic TS, whereas it involved a disrotation for a photochemical eletrocyclic reaction.
References in Exercise 3
- ↑ D. Suárez, E. Iglesias, T. L. Sordo, J. A. Sordo, J. Phys. Org. Chem., 1996, 9, 17–20, DOI:<17::AID-POC749>3.0.CO;2-D 10.1002/(SICI)1099-1395(199601)9:1<17::AID-POC749>3.0.CO;2-D .
- ↑ R. B. Woodward, R. Hoffman, Angew. Chem. Int. Ed. Engl., 1969, 8, 781–853, DOI:10.1002/anie.196907811 .
- ↑ F. Monnat, P. Vogel, V. M. Rayon, J. A. Sordo, J. Org. Chem., 2002, 67, 1882-1889, DOI:10.1021/jo010998w .
- ↑ S. M. Mukherji, S. P. Singh, R. P.Feynman,R. Dass, Organic Chemistry Vol I, New Age International, New Delhi, India, 2010.
- ↑ 5.0 5.1 J. Clayden, N. Greeves, S. Warren, P. Wothers, Organic Chemistry, Oxford University Press Inc., New York, 2001.