Rep:Xiaojie102820
Module 3
In this page, I will characterise transition structures on potential energy surfaces for the Cope rearrangement and Diels Alder cycloaddition reactions.
The Cope Rearrangement Tutorial
In this section,I will use the Cope rearrangement of 1,5-hexadiene as an example.And my objectives are to locate the low-energy minima and transition structures on the C6H10 potential energy surface, to determine the preferred reaction mechanism.

Molecule1,5-hexadiene
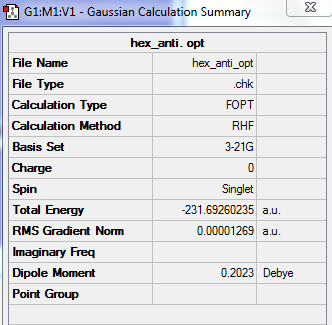
HF/3-21G Optimised (Anti 1) 1,5-hexadiene
test molecule |
Summary Table
Point group Analysis
General Information
| Energy | -231.69260235a.u. |
| Point Group | C2 |
By comparing to the Appendix 1, this structure should be Anti-1.
Output File
Item Value Threshold Converged?
Maximum Force 0.000027 0.000450 YES
RMS Force 0.000008 0.000300 YES
Maximum Displacement 0.000825 0.001800 YES
RMS Displacement 0.000314 0.001200 YES
Predicted change in Energy=-2.682036D-08
Optimization completed.
-- Stationary point found.
File Link
The log. file of HF/3-21G optimised (anti-1) 1,5-hexadiene
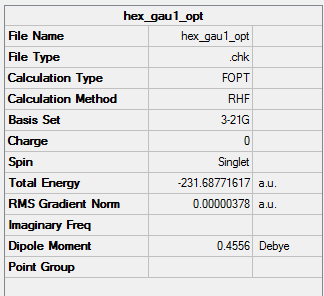
HF/3-21G Optimised (Gauche1) 1,5-hexadiene
Now I will optimised 1,5-hexadiene molecule with a "gauche" linkage for the central four C atoms. With the previous knowledage from the conformational analysis course(2nd year), the gauche form should be lower in the energy. Unlike small straight molecules which prefer app(anti-periplanar) due to large orbital overlap, the large molecules shift balance to the gauche conformation(given highly proportion of gauche form in large molecules). This incursion of gauche forms is due to the longer distances favouring folding of the chain back upon itself, and hence setting up van der Waals attractions.
test molecule |
Summary Table
Point Group Analysis
General Information
| Energy | -231.68771617a.u. |
| Point Group | C2 with a twofold symmetry axis |
By checking the Appendix 1, this structure is Gauche-1.
Now we can compare the final energy of this two structures. The 'gauche' linkage is 0.00488618a.u.(about3.066kcal/mol) higher in energy than the 'anti' linkage. This result is different from my prediction. I think there is overestimation of the enetgy for gauche form when we using the 3-21G basis set.
Output File
Item Value Threshold Converged?
Maximum Force 0.000012 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000466 0.001800 YES
RMS Displacement 0.000111 0.001200 YES
Predicted change in Energy=-1.790991D-09
Optimization completed.
-- Stationary point found.
File Link
Log.file of HF/3-21G optimised (Gauche-1) structure
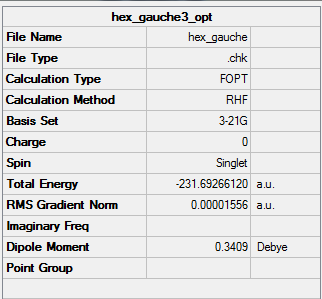
Lowest energy conformation-(gauche3)
Prediction: Based on the results I got from above, I think the anti/gauche/anti conformer might be the lowest energy conformation of 1,5-hexadiene. In order to check the result, I need to optimised the guess structure.
test molecule |
Summary Table
Point Group Analysis
| Energy | -231.69266120a.u. |
| Point Group | C1 |
The energy I get is -231.69266120a.u. which is lower than the above two structures(check!).
Comparing the structure in the Appendix table, the conformer which I predict is Gauche-3.
Output File
Item Value Threshold Converged?
Maximum Force 0.000044 0.000450 YES
RMS Force 0.000009 0.000300 YES
Maximum Displacement 0.001317 0.001800 YES
RMS Displacement 0.000491 0.001200 YES
Predicted change in Energy=-3.610321D-08
Optimization completed.
-- Stationary point found.
File Link
LOG. file of HF/3-21G optimised (gauche-3)structure
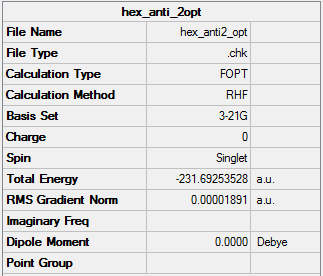
HF/3-21G Optimised (Anti2) 1,5-hexadiene
test molecule |
Summary Tble
Point Group Analysis
The energy I got is -231.69253528a.u. which is very colse to the value in the table(-231.69254a.u.).
The point group is Ci(identical to the one in table).
Output File
Item Value Threshold Converged?
Maximum Force 0.000060 0.000450 YES
RMS Force 0.000010 0.000300 YES
Maximum Displacement 0.000476 0.001800 YES
RMS Displacement 0.000171 0.001200 YES
Predicted change in Energy=-2.037252D-08
Optimization completed.
-- Stationary point found.
File Link
LOG. file of the HF/3-21G optimised (Anti-2) conformer
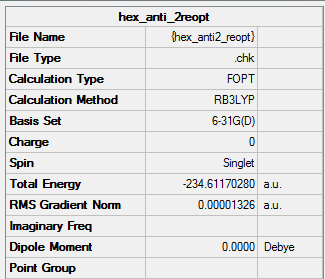
B3LYP/6-31G* Optimised (Anti2) 1,5-hexadiene
This time, I will optimise the anti 2 structure at a higher level of theory.
test molecule |
Summary Table
Point Group
The energy decreased to -234.61170280a.u. And the point group is Ci.
Output file
Item Value Threshold Converged?
Maximum Force 0.000015 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000219 0.001800 YES
RMS Displacement 0.000079 0.001200 YES
Predicted change in Energy=-1.588886D-08
Optimization completed.
-- Stationary point found.
geometry Comparison
| No. | HF/3-21G | B3LYP/6-31* |
|---|---|---|
| c=c Bond length | 1.31613Å | 1.33352Å |
| C13-C10-C7 | 126.8060C | 125.2870C |
The bond length of the carbon-carbon double bond is lengthened, while, the bond length of the carbon-carbon single bond is shortened on the B3LYP/6-31G* calculation.
The B3LYP/6-31G* method has a better basis set than the HF/3-21G method,gives a better aggrement with the experiment value.
File link
LOG. file of B3LYP/6-31G* Optimised (Anti2)conformer
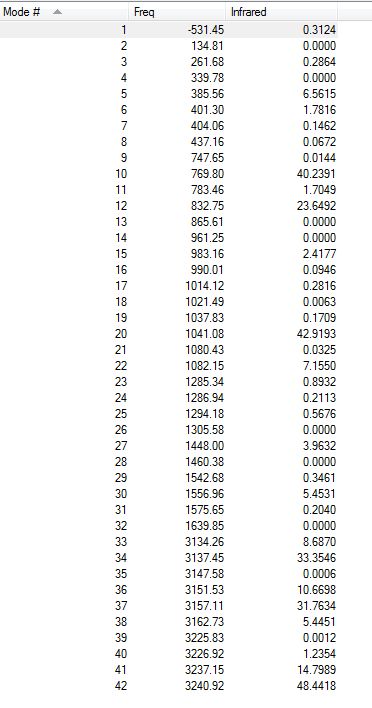
Frequency Analysis of B3LYP/6-31G* Optimised (Anti2) 1,5-hexadiene
The final energies given represent the energy of th molecule on the bare potential energy surface. In order to compare these energies with experimental value, the frequency calculation is required.
vibrational frequencies
It is clear that there is no imaginary frequencies, only real ones which confirms it is a minimum(the frequency analysis is essentially the second derivative of the potential energy surface, if the frequencies are all positive then we have a minimum).
IR spectrum
File
Sum of electronic and zero-point Energies= -234.469212 Sum of electronic and thermal Energies= -234.461856 Sum of electronic and thermal Enthalpies= -234.460912 Sum of electronic and thermal Free Energies= -234.500821
File link
Frequency file of anti2 conformer
"Chair" and "Boat" Transition Structures
In this section I will set up a transition structure optimization.
allyl fragment
HF/3-21G optimised allyl fragement
test molecule |
Summary Table
Output file
Item Value Threshold Converged?
Maximum Force 0.000048 0.000450 YES
RMS Force 0.000018 0.000300 YES
Maximum Displacement 0.000141 0.001800 YES
RMS Displacement 0.000070 0.001200 YES
Predicted change in Energy=-1.277267D-08
Optimization completed.
-- Stationary point found.
File Link
Chair transition state-guess structure
Now we are going to optimise the transition state. Actually,the transition states correspond to saddle points with one negative second derivative on the potential energy surface. So in order to locate the transition state we can find a point with one negative second derivative(which is one imaginary frequency in this experiment). The reason why transition state optimisation is more difficult than that of minimum is that a successful search should start off in a region where the reaction coordinate already has a negative curvature.(which I did at second year lab: search for a transition state should start near the transition state!)
Optimisation of chair-ts in 3-21G
test molecule |
Summary Table
Output file
Item Value Threshold Converged?
Maximum Force 0.000024 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.001401 0.001800 YES
RMS Displacement 0.000201 0.001200 YES
Predicted change in Energy=-1.246508D-07
Optimization completed.
-- Stationary point found.
File Link
Log. file of 3-21G optimised chair ts
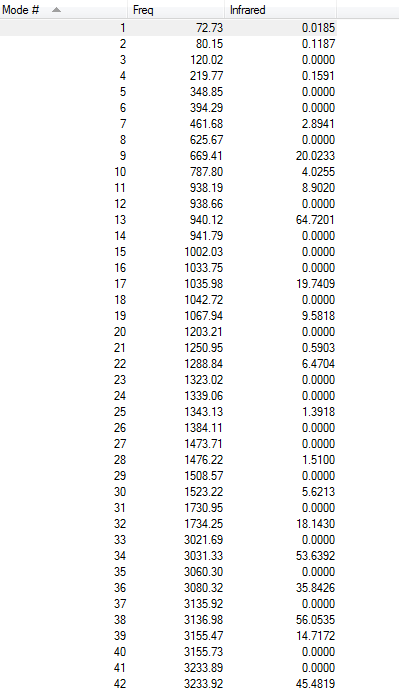
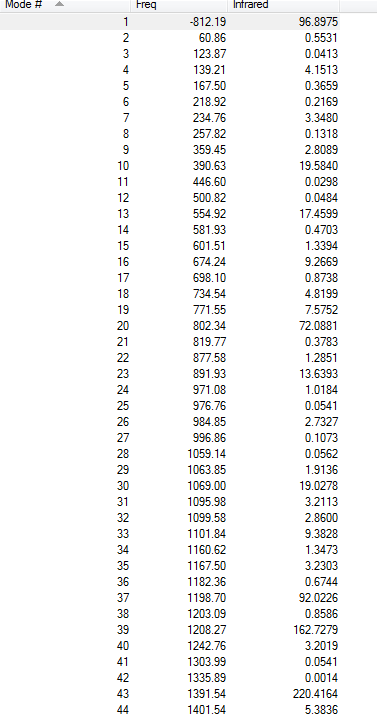
Frequency Analysis
Vibrational Frequency
The frequency calculation gives an(only one) imaginary frequency of magnitude 817.98 cm-1. It means that the guess transition state is reasonable(reason explained above).
Animation
File
Sum of electronic and zero-point Energies= -231.466702 Sum of electronic and thermal Energies= -231.461342 Sum of electronic and thermal Enthalpies= -231.460398 Sum of electronic and thermal Free Energies= -231.495208
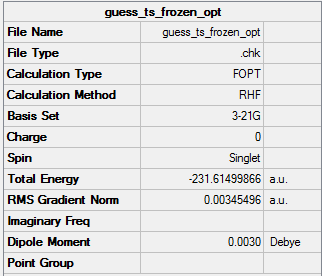
Optimisation of chair-ts by using the frozen coordinate method
test molecule |
CHK file
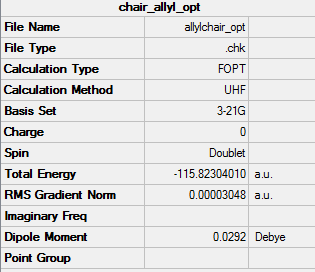
The optimized structure looks a lot like the transition I got by using 3-21G. However, bond forming/breaking distances are fixed to 2.2 Å.
Summary table
The final energy here is -231.61499866a.u.which is greater than the one in the HF/3-21G.
Output file
Item Value Threshold Converged?
Maximum Force 0.000011 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000512 0.001800 YES
RMS Displacement 0.000088 0.001200 YES
Predicted change in Energy=-3.015680D-08
Optimization completed.
-- Stationary point found.
File Link
File of frozen coordinate method optimised chair ts
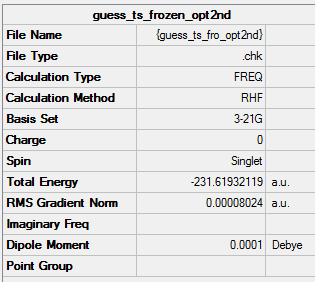
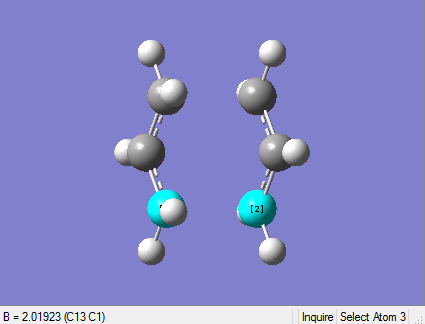
Reoptimisation after Redundant Coord Editor
test molecule |
Summary Table
Output File
Item Value Threshold Converged?
Maximum Force 0.000051 0.000450 YES
RMS Force 0.000013 0.000300 YES
Maximum Displacement 0.001240 0.001800 YES
RMS Displacement 0.000244 0.001200 YES
Predicted change in Energy=-6.076100D-07
Optimization completed.
-- Stationary point found.
File Link
File of reoptimised chair ts after RCE
chk
bond forming/bond breaking bond length is 2.01923 Å
Comparison Table
| hf/3-21g | After RCE |
|---|---|
| 2.02049Å | 2.01923Å |
Boat transition state
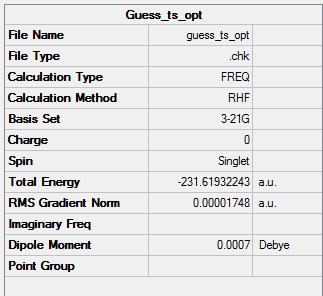
We are going to optimise the boat transition state using QST2 method. It requires that the reactants and products are numbered in the same way.
Reactant/product-QST2 calculation
test molecule |
Summary Table
Output file
Item Value Threshold Converged?
Maximum Force 0.000094 0.000450 YES
RMS Force 0.000016 0.000300 YES
Maximum Displacement 0.001626 0.001800 YES
RMS Displacement 0.000525 0.001200 YES
Predicted change in Energy=-4.528634D-08
Optimization completed.
-- Stationary point found.
File Link
File of QST2 optimised reactant
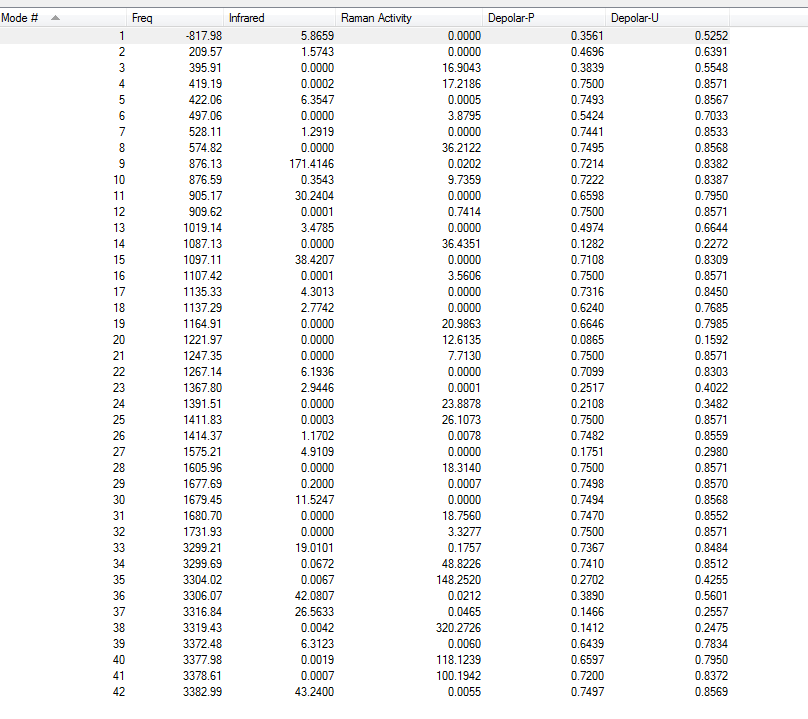
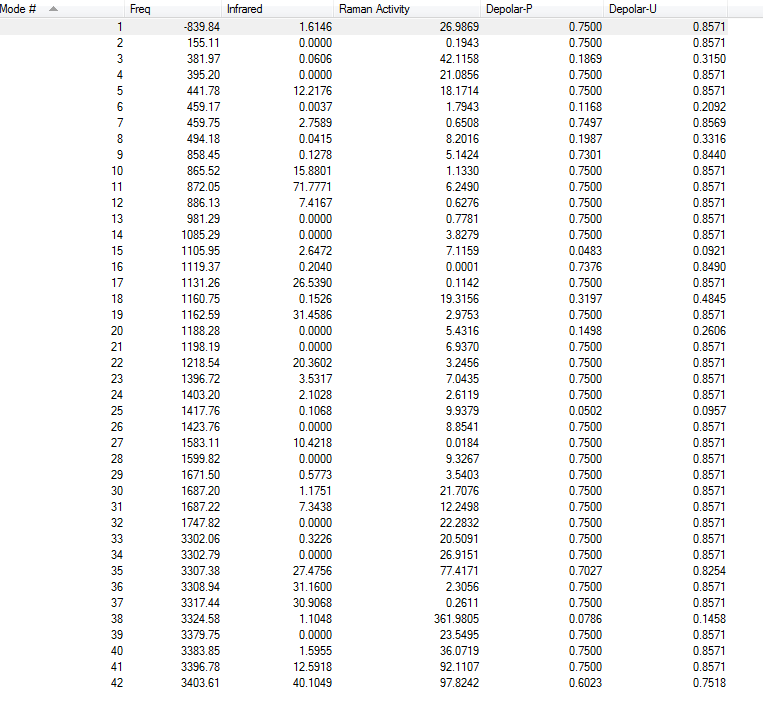
Vibrational Analysis
There is only one imaginary frequency of magnitude of 839.84cm-1(confirms it is a transition state).
Animation
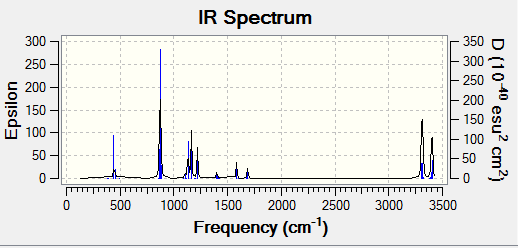
IR Spectrum
Output File
Sum of electronic and zero-point Energies= -231.450926 Sum of electronic and thermal Energies= -231.445297 Sum of electronic and thermal Enthalpies= -231.444352 Sum of electronic and thermal Free Energies= -231.479772
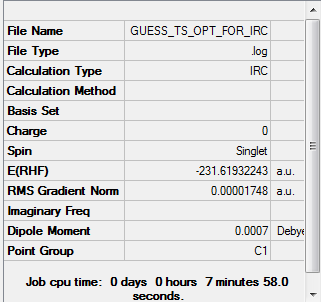
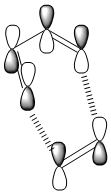
IRC Analysis of Chair Transition state
Since I have known the optimised chair and boat structure, here is a problem raised: Which conformers of 1,5-hexadiene do they connect? And I find it is hard to predict which conformer the reaction paths from the transitions structures will lead to. So in this section, I will run the IRC method which allows me to follow the minimum energy path from a transition structure down to its local minimum on a potential energy surface.
irc pathway
There are 27 intermediate geometries. According to the IRC pathway diagram ,we start from the transition state(red circled point on the graph) and the energy goes down towards the minimum with the deepest gradient.However, it hasn't reached the minimum geometry and it stopped! So In order to get the minimum, I can take the last point on the IRC and run a normal minimization(optimisation).
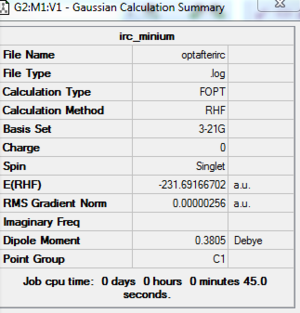
Run minimization
test molecule |
Summary Table
Point Group
After the optimisation, the final gradient decreased to 0.00000256. In addition,the final energy is -231.69167a.u. and the point group is C2. Now we can look up the Appendix table, the structure we get is Gauche-2!(the conformer that chair and boat connect).
Optimisation of Chair and Boat by using B3LYP/6-31G*
Finally I will calculate the activation energies for our reaction via both transition structures.
B3LYP/6-31G* optimised Chair transition state
test molecule |
Summary Table
Output File
Item Value Threshold Converged?
Maximum Force 0.000024 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000829 0.001800 YES
RMS Displacement 0.000115 0.001200 YES
Predicted change in Energy=-7.523575D-08
Optimization completed.
-- Stationary point found.
File Link
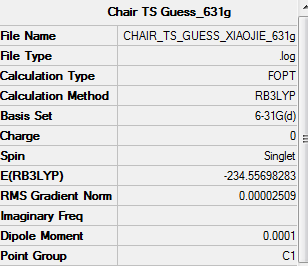
B3LYP/6-31G* optimised Chair ts log. file B3LYP/6-31G optimised chair ts
B3LYP/6-31G* optimised Boat transition state
test molecule |
Summary Table
Output File
Item Value Threshold Converged?
Maximum Force 0.000304 0.000450 YES
RMS Force 0.000074 0.000300 YES
Maximum Displacement 0.001756 0.001800 YES
RMS Displacement 0.000780 0.001200 YES
Predicted change in Energy=-1.694834D-06
Optimization completed.
-- Stationary point found.
File Link
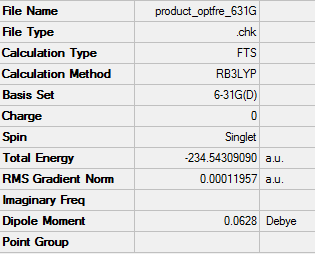
Log. file of B3LYP/6-31G optimised Boat ts
Frequency analysis of B3LYP/6-31G*optimised transition state
Chair TS
Output File
Sum of electronic and zero-point Energies= -234.414931 Sum of electronic and thermal Energies= -234.409010 Sum of electronic and thermal Enthalpies= -234.408066 Sum of electronic and thermal Free Energies= -234.443817
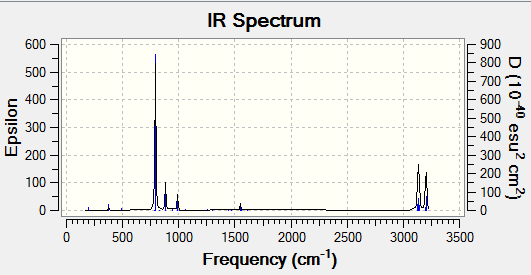
IR spectrum
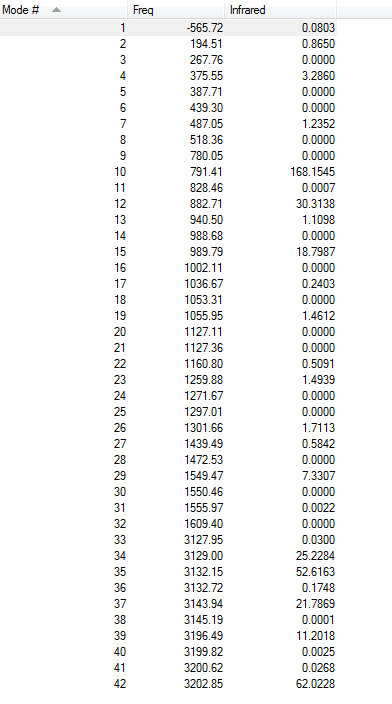
Vibration frequency
It is clear that there is only one imaginary frequency(-565.72) which again confirms it is a transition state.
Vibrational animation
File Link
Frequency output file of 6-31G* optimised chair ts
Boat TS
Output File
Sum of electronic and zero-point Energies= -234.402318 Sum of electronic and thermal Energies= -234.395985 Sum of electronic and thermal Enthalpies= -234.395041 Sum of electronic and thermal Free Energies= -234.431729
Vibrational Frequency
IR Spectrum
File Link
Frequency output file of optimised boat ts
Result Table
Summary of energies (in hartree)
| HF/3-21G | B3LYP/6-31G* | |||||
|---|---|---|---|---|---|---|
| Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | |
| at 0 K | at 298.15 K | at 0 K | at 298.15 K | |||
| Chair TS | -231.61932243 | -231.466702 | -231.461342 | -234.55698283 | -234.414931 | -234.409010 |
| Boat TS | -231.60280245 | -231.450926 | -231.445297 | -234.54309090 | -234.402318 | -234.395985 |
| Reactant (anti2) | -231.69253528 | -231.539539 | -231.532565 | -234.61170280 | -234.469212 | -234.461856 |
*1 hartree = 627.509 kcal/mol
Summary of activation energies (in kcal/mol)
| HF/3-21G | HF/3-21G | B3LYP/6-31G* | B3LYP/6-31G* | Expt. | |
| at 0 K | at 298.15 K | at 0 K | at 298.15 K | at 0 K | |
| ΔE (Chair) | 45.70 | 44.69 | 34.06 | 33.16 | 33.5 ± 0.5 |
| ΔE (Boat) | 55.60 | 54.76 | 41.98 | 41.33 | 44.7 ± 2.0 |
Ater comparing the activation energy of two basis set, it is clear that although the geometries are reasonably similar, energy differences are markedly different! Moreover, activation energies calculated at BYLYP/6-31G* are in good aggrement with the experiment value. Finally, the activation energy of the chair ts is about10kJ/mol lower than that of the boat transition state(small barrier!). So the reaction will more likely to proceed via a chair transition state.
The Diels Alder Cycloaddition
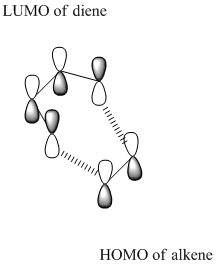
In this exercise, I will characterise transition structures using the method I have learned in the tutorial part. The Diels–Alder reaction is an organic chemical reaction (specifically, a cycloaddition) between a conjugated diene and a substituted alkene,to form a substituted cyclohexene system. The HOMO-LUMO overlap can be used to perdict whether the reaction occur or not.
cis butadiene
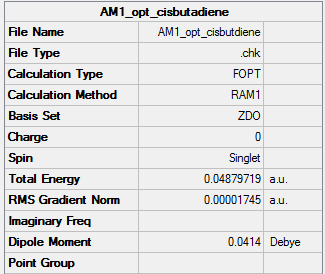
AM1 optimised ciabutadiene
test molecule |
Summary Table
| Energy | 0.04879719a.u. |
| Point group | C2v |
Output File
Item Value Threshold Converged?
Maximum Force 0.000030 0.000450 YES
RMS Force 0.000011 0.000300 YES
Maximum Displacement 0.000360 0.001800 YES
RMS Displacement 0.000162 0.001200 YES
Predicted change in Energy=-9.691168D-09
Optimization completed.
-- Stationary point found.
File Link
File Link of AM1 optimised cisbutadiene
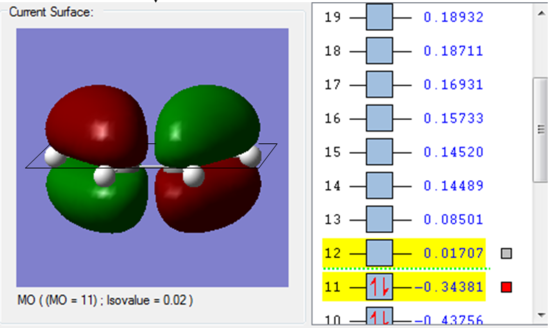
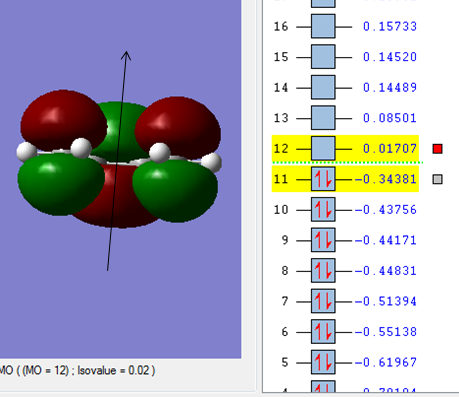
Molecule orbital
| HOMO | LUMO |
 |
|
| It is clear that the HOMO orbital of cic-butadiene is asymmetric(a) with repect to the plane. | While the LUMO orbital is symmetric(s) with respect to the plane. |
Transition State geometry for the prototype reaction
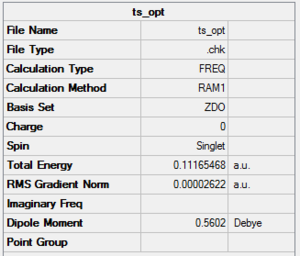
Optimisation of the ts
test molecule |
Summary Table
Point group
General information
| Energy | 0.11165468a.u. |
| Point Group | Cs |
Output file
Item Value Threshold Converged?
Maximum Force 0.000065 0.000450 YES
RMS Force 0.000011 0.000300 YES
Maximum Displacement 0.001005 0.001800 YES
RMS Displacement 0.000240 0.001200 YES
Predicted change in Energy=-2.489584D-08
Optimization completed.
-- Stationary point found.
Geometry Information
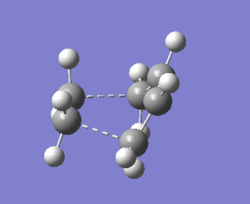
| partly formed sigma C-C bond | SP3 C-C bond | SP2 C-C bond | Van Der Waal radius of C atom |
|---|---|---|---|
| 2.1195 Å | 1.54000 Å[1] | 1.35520 Å[2] | 1.70 Å[3] |
The new C-C sigma bond length is 2.1195 Å. It is shorter than the twice of Van Der Waal redius(1.70*2=3.40 Å) which indicates there do has bonds forming between the terminal carbons. However, It is longer than the SP3 and SP2 C-C bond which means that the new sigma bond in the transition state is just partly formed.
File Link
File of optimised transition state
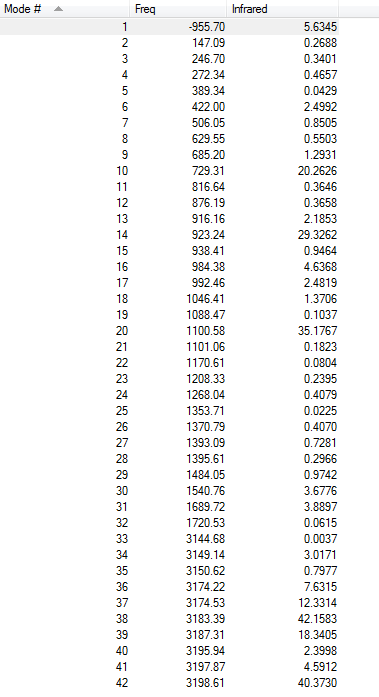
Vibrational frequency
There is only one imaginary frequency of magnitude 955.70cm-1, again indicates it is a transition state.
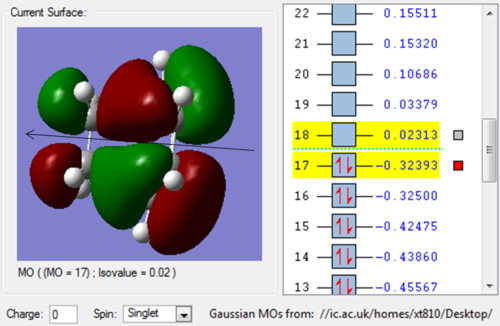
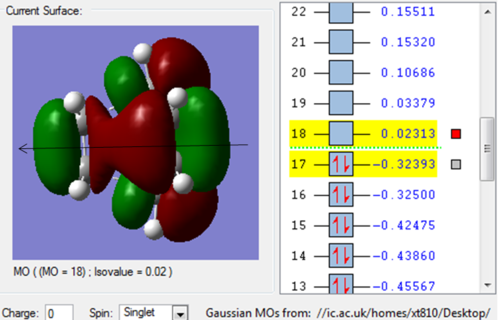
Molecule Orbital
| HOMO | LUMO |
 |
|
| The HOMO is asymmetric(a) with respect to the plane. | While the LUMO is symmetric(s). |
Conclusion
The symmetry of the HOMO at transition state can be found from the MO diagram above. It is clear that the HOMO is asymmetric.The LUMO of ethylene and the HOMO of the butadiene are both a. Thurs it is the HOMO-LUMO pairs of orbital that interact. The reaction is allowed because the HOMO of butadiene can interact with the LUMO of the ethylene;in other words, the HOMO(butadiene)and LUMO(ethylene) have the same symmetry.
| ethylene | butadiene |
|---|---|
 |
 |
| asymmetric | asymmetric |
HOMO-LUMO interact-chemdraw(HOMO Of cis butadiene and LUMO of ethylene)
Approach with the ethylene under diene.
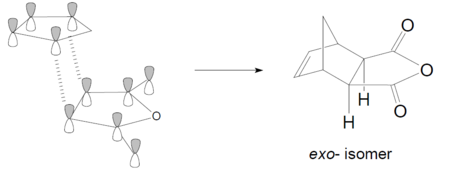
The regioselectivity of the Diels Alder Reaction
Exo transition state
Optimisation of exo-ts
test molecule |
Summary Table
Point group
General Information
| Energy | -0..5041985a.u. |
| point group | Cs |
Output File
Item Value Threshold Converged?
Maximum Force 0.000002 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000075 0.001800 YES
RMS Displacement 0.000015 0.001200 YES
Predicted change in Energy=-1.459715D-10
Optimization completed.
-- Stationary point found.
Vibrational frequency
There is only one imaginary frequency of magnitude of 812.19cm-1, again confirms it is a transition state.
File link
File of optimised exo-transition state
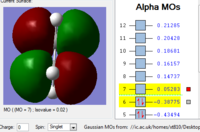
Molecule orbital Analysis
| HOMO | LUMO |
 |
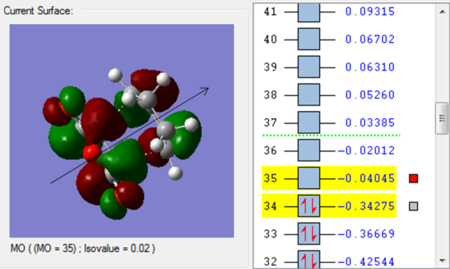
|
| The HOMO is asymmetric w.r.t the plane | The LUMO is asymmetric w.r.t the plane as well |
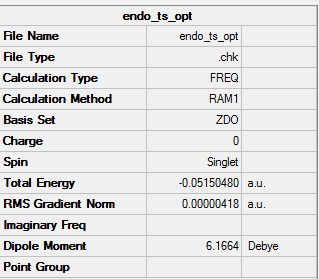
ENDO transition state
Optimisation of endo ts
test molecule |
Summary Table
Point group
General Information
| point group | Cs |
| Energy | -0.05150480 a.u. |
Output File
Item Value Threshold Converged?
Maximum Force 0.000010 0.000450 YES
RMS Force 0.000002 0.000300 YES
Maximum Displacement 0.000194 0.001800 YES
RMS Displacement 0.000054 0.001200 YES
Predicted change in Energy=-2.030039D-09
Optimization completed.
-- Stationary point found.
File Link
File link of optimised endo transition state
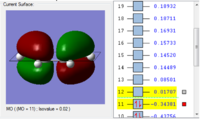
Molecule Orbital Analysis
| HOMO | LUMO |
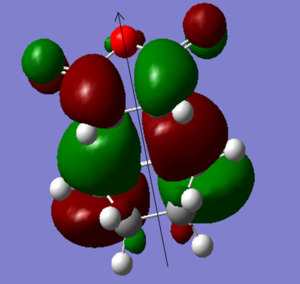 |
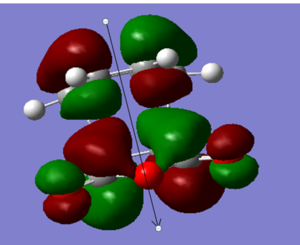
|
| The HOMO is asymmetric with respect to the plane | The LUMO is asymmetric with respect to the plane as well |
Discussion
| ts | C-C space | partly formed C-C bond | other C-C bond |
| exo | 2.94510Å | 2.17040Å | 1.48819Å |
| endo | 2.89215Å | 2.16232Å | 1.48923Å |
Structure comparison
For the exo transition state, the large maleic anhydride group is located near to the -CH2-CH2- fragment which leads to high steric hindrance. so the exo-ts is higher in energy(more strained). However, this steric hindrance does not exists for the endo form as the maleic anhydride group is located far away from the beidging carbon fragments. Consequently, the endo adduct will be the major product under kinetic control due to the lower activation barrier.
HOMO-LUMO overlap
The reaction is allowed due to perfect interaction of the HOMO-LUMO.
secondary orbital overlap effect-MO overlap
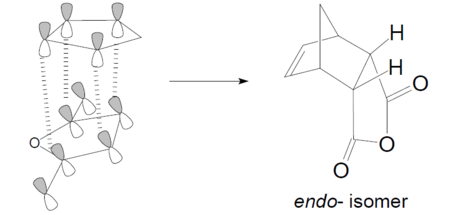
The secondary orbital overlap effect is defined as the positive overlap of the nonactive frontier molecule orbital of a pericyclic reaction.
According to the diagram above, it is clear that the P orbitals on the carbonyl carbon atom can overlap with the p orbitals of carbon on diene which leads to the stabilisation of the endo-transition state. While in exo-form, there is no such interaction.
Further discussion
Ans: The conditions of the solvent used is neglect when we run the calculation.
References
- ↑ Fox, Marye Anne; Whitesell, James K. (1995). Organische Chemie: Grundlagen, Mechanismen, Bioorganische Anwendungen. Springer.
- ↑ Fox, Marye Anne; Whitesell, James K. (1995). Organische Chemie: Grundlagen, Mechanismen, Bioorganische Anwendungen. Springer. .
- ↑ ^ a b c Bondi, A. (1964). "Van der Waals Volumes and Radii". J. Phys. Chem. 68 (3): 441–51.[1]