Rep:Transition states (st4215): Exercise 2
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole
The reaction of cyclohexadiene and 1,3-dioxole is also a Diels-Alder reaction, in which cyclohexadiene is the diene and 1,3-dioxole is the dienophile. However, unlike the simple Diels-Alder reaction between butadiene and ethylene, this reaction is slightly more complex - 1,3-dioxole can approach the diene at different orientations, leading to the formation of the endo- and exo- Diels-Alder products. This can be seen in the reaction scheme below:
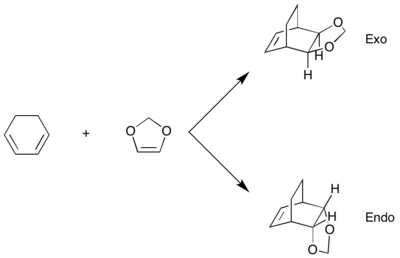
The endo- product is formed from a transition state where the substituents on the dienophile point towards the π system of the diene, while the exo- product is formed from a transition state where the substituents are pointing away.
In this exercise, we take a closer look at a different aspect of the Diels-Alder reaction - whether it is normal or inverse electron demand. We also consider the Diels-Alder reaction when an unsymmetrical diene is involved, and compare the endo- and exo- DA reactions in terms of which is more kinetically or thermodynamically favourable. In the process, we confirm that the orbital symmetry requirements as elucidated in Exercise 1 still apply, and illustrate that there are differences between the PM6 and B3LYP calculation methods, with one being more suitable than the other in this case.
Calculations
Calculations were performed at both the PM6 and B3LYP/6-31G(d) level. Method 2 (see tutorial) was used to locate the transition state.
The optimised molecules can be seen here:
| Calculation method | Reactants | Endo | Exo | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclohexadiene | 1,3-dioxole | Transition state | Product | Transition state | Product | |||||||||||||
| B3LYP | ||||||||||||||||||
| PM6 | Cyclohexadiene (PM6) | 1,3-dioxole (PM6) | Endo-TS (PM6) IRC file |
Endo-product (PM6) | Exo-TS (PM6) IRC file |
Exo-product(PM6) | ||||||||||||
Molecular orbitals
Computed MOs
Using your MO diagram for the Diels-Alder reaction, locate the occupied and unoccupied orbitals associated with the DA reaction for both TSs by symmetry. Find the relevant MOs and add them to your wiki (at an appropriate angle to show symmetry).
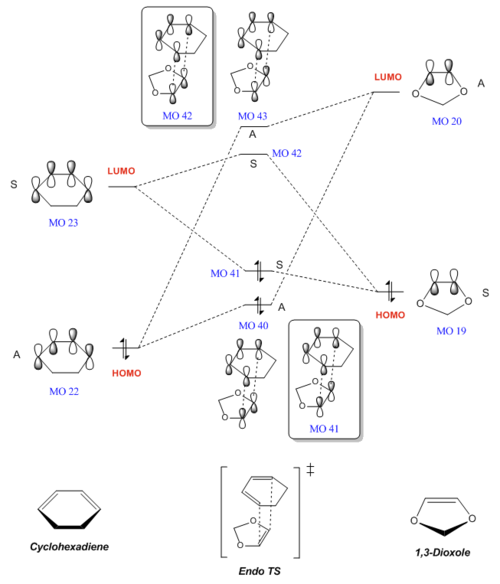
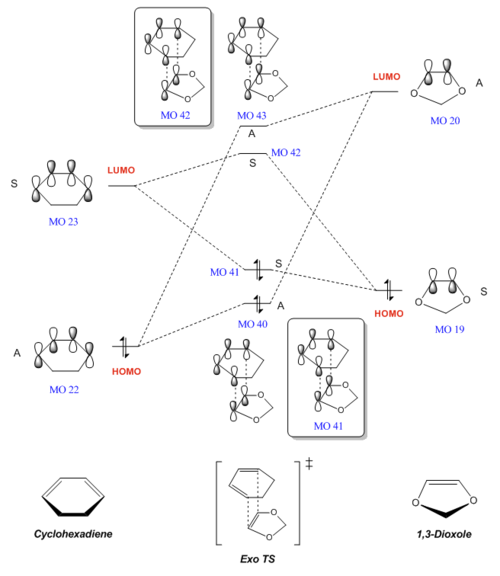
The MOs involved in this reaction - the HOMOs and LUMOs for the reactants (cyclohexadiene and 1,3-dioxole), as well as the 4 MOs that these produce in each of the endo and exo transition states - are shown below. As determined in Exercise 1, which is also a Diels-Alder reaction, only orbitals of the same symmetry are able to interact. This is illustrated in the table below, where the symmetry or antisymmetry of each orbital can be clearly seen.
| Symmetry | Reactants | Transition state (Endo) | Transition state (Exo) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclohexadiene | 1,3-Dioxole | |||||||||||||||||
| Antisymmmetric | MO 22 (HOMO) |
MO 20 (LUMO) |
MO 40 |
MO 43 |
MO 40 |
MO 43 | ||||||||||||
| Symmetric | MO 23 (LUMO) |
MO 19 (HOMO) |
MO 41 |
MO 42 |
MO 41 |
MO 42 | ||||||||||||
It is important to note that these MOs were generated from the B3LYP and not the PM6 calculation. While both calculation methods were used, the B3LYP calculation proved to be a better choice in visualising the MOs as it allowed us to see the symmetry of the orbitals more clearly, and corresponded more closely to the graphical representation of the orbitals in the MO diagram (see next section).
MO diagrams
Construct a new MO diagram using these new orbitals, adjusting energy levels as necessary. Is this a normal or inverse demand DA reaction?
MO diagrams for both the endo- and exo- Diels-Alder reactions were constructed as shown below. Energy levels of the MOs have been adjusted to reflect their actual values, as calculated in the B3LYP calculation.
| Endo- Diels-Alder | Exo- Diels-Alder |
|---|---|
|
|
From the MO diagrams above, we can conclude that both the endo- and exo- reactions are inverse demand DA reactions. In contrast to a normal electron demand DA reaction, where the diene is electron rich with a high LUMO and the dienophile is electron poor with a low HOMO, cyclohexadiene (the diene) is electron poor with a low HOMO and 1,3-dioxole (the dienophile) is electron rich with a high LUMO. Hence we can classify these reactions as inverse demand.
This is likely due to the nature of the dienophile, 1-3-dioxole, which has an electron-donating O atom. The lone pairs on the two adjacent O atoms are able to donate electron density into the double bond, making the dienophile more electron rich and raising the energy of its MOs, including its LUMO. In contrast, cyclohexadiene does not have any electron-donating substituents and is relatively electron poor. Hence, considering the Frontier Molecular Orbitals (FMOs), the LUMOdienophile is relatively high-energy and closer in energy to the low-energy HOMOdiene, as compared to HOMOdienophile and LUMOdiene. Thus the interaction between the LUMOdienophile and HOMOdiene is the strongest and dominates in this reaction, causing it to be inverse demand.[1]
Nf710 (talk) 00:01, 8 November 2017 (UTC) Good understanding of the demand of the electron of a DA reaction
Energy analysis
Tabulate the energies and determine the reaction barriers and reaction energies (in kJ/mol) at room temperature (the corrected energies are labelled "Sum of electronic and thermal Free Energies", corresponding to the Gibbs free energy). Which are the kinetically and thermodynamically favourable products? Look at the HOMO of the TSs. Are there any secondary orbital interactions or sterics that might affect the reaction barrier energy?
Free energies
The free energy of the reactants as well as the endo- and exo- Diels-Alder transition states and products are tabulated below. The reaction barriers and energies are also calculated.
| Energy at 298 K | ||||
|---|---|---|---|---|
| B3LYP | PM6 | |||
| Hartree | kJmol-1 | Hartree | kJmol-1 | |
| Reactants | ||||
| Cyclohexadiene | -233.32 | -6.1259 x 105 | 0.11688 | 306.86 |
| 1,3-dioxole | -267.07 | -7.0119 x 105 | -0.052279 | -137.26 |
| Total reactants | -500.39 | -1.3138 x 106 | 0.064598 | 169.60 |
| Endo- Diels-Alder | ||||
| Transition state | -500.332 | -1.3136 x 106 | 0.13794 | 362.17 |
| Product | -500.419 | -1.3138 x 106 | 0.037807 | 99.26 |
| Reaction barrier (Ea) | 0.0609 | 160 | 0.0733 | 193 |
| Reaction energy (ΔG) | -0.0257 | -67.4 | -0.0268 | -70.3 |
| Exo- Diels-Alder | ||||
| Transition state | -500.329 | -1.3136 x 106 | 0.13890 | 364.68 |
| Product | -500.417 | -1.3138 x 106 | 0.037977 | 99.71 |
| Reaction barrier (Ea) | 0.0639 | 168 | 0.0743 | 195 |
| Reaction energy (ΔG) | -0.0243 | -63.8 | -0.0266 | -69.9 |
^Note that all values from the calculations are reported to 5 s.f. unless more decimal places are needed to differentiate between endo- and exo- geometries. Derived quantities (reaction barrier and reaction energy) are reported to 3 s.f.
Thermodynamic and kinetic products
From the table above, we can conclude that the endo- product is both the kinetic and thermodynamic product. This is because it has a lower reaction barrier, meaning that less activation energy is required for the reaction, making it the kinetic product. The endo- product also has a lower reaction energy (ΔG), meaning that the endo- product is lower in energy and more stable than the exo- product, hence making it the thermodynamic product.
This is in contrast to most Diels-Alder reactions, in which the exo- product is the thermodynamic product as it is less sterically hindered and more stable, while the endo- product is the kinetic product due to stabilising orbital interactions in the transition state.[2] However, the difference for this reaction can be explained.
As seen in the jmols below, the endo- TS indeed has stabilising secondary orbital interactions between the p orbitals of O in 1,3-dioxole and the p orbitals of C in cyclohexadiene, which are absent in the exo- TS. The presence of these interactions lower the energy of the transition state, hence lowering the reaction barrier and making the endo-product the kinetic product.
Comparing the endo- and exo- products, we can also see that these stabilising orbital interactions are also in present in the endo- product. Additionally, the endo- product is less sterically hindered compared to the exo-product, in which there is steric clash between the hydrogens which are pointing towards each other. These two factors cause the energy of the endo- product to be lower than that of the exo- product, hence lowering the reaction energy and making the endo- product the thermodynamic product as well.
| Endo | Exo | |||||
|---|---|---|---|---|---|---|
| Transition state | ||||||
| Product |
Nf710 (talk) 00:05, 8 November 2017 (UTC) This is an excellent understanding of the thermodynamics and kenetics of the reaction. You could have improved your answer by addding a drawing showing the steric clashes.
Conclusion
As per Exercise 1, MO calculations and visualisation for this Diels-Alder reaction prove that only orbitals with the same symmetry can overlap and interact, hence showing that this is not unique to a singular reaction. A closer examination of the MO diagram also allows us to determine that this reaction is an inverse electron demand DA reaction, due to the presence of electron-donating groups on the dienophile (1,3-dioxole) which raises the energy of its LUMO. Analysing the energy levels of the reactants, TSs and products also reveal that the endo- product is both the kinetic and thermodynamic product, due to greater stability of the transition state (due to stabilising secondary orbital interactions) and the final product (due to lesser steric clash).
