Rep:Transition States and Reactivity Exercise3 ZWL115
Exercise 3
Reaction Scheme
O-Xylylene can react with SO2 via 2 different Diels-Alder cycloadditions and a chelotropic pathway. These pathways are illustrated in Figure. 5.
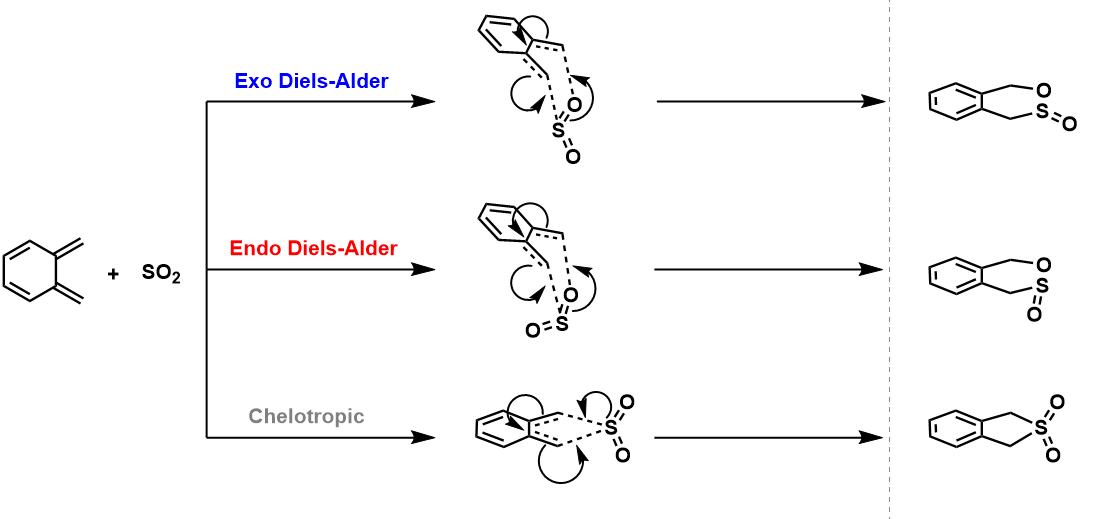
| Reactants | Transition States | Products | ||||||
|---|---|---|---|---|---|---|---|---|
(Please check and understand the JMol code that you're using. You're asking for a particular MO number when you mean frame number Tam10 (talk) 12:10, 7 March 2018 (UTC))
Reaction Coordinate
| Endo | Exo | Chelotropic |
|---|---|---|

|

|

|
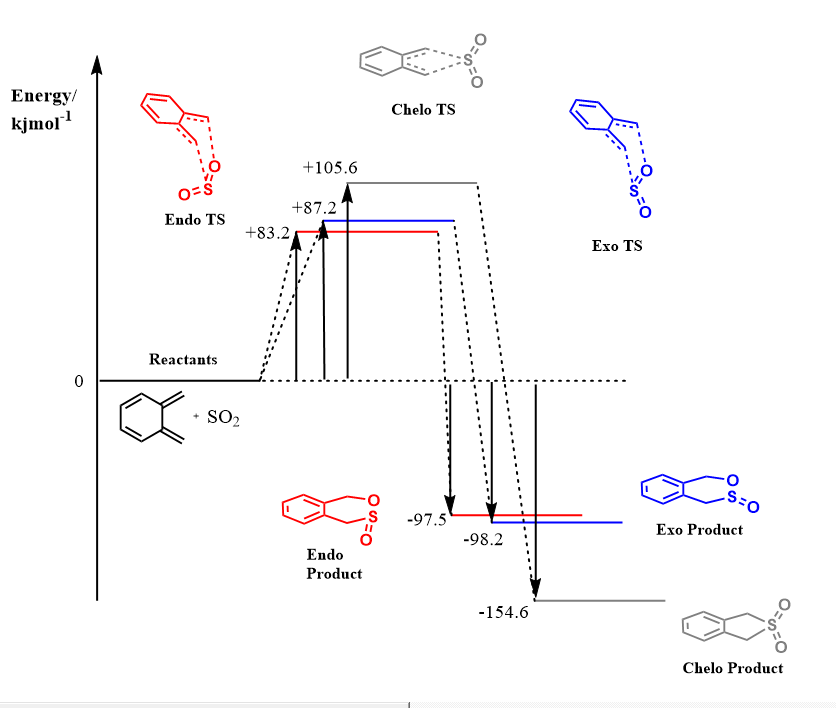
The instability of Xylylene can be rationalised using Hückel's Molecular Orbital Theory. According to the theory, a compound is stable when all of its bonding orbitals are filled and no electrons fill the anti-bonding orbitals. This is especially true in aromatic compounds, where 2 electrons fill the lowest energy molecular orbital and 4 electrons fill the subsequent degenerate pair of orbitals (the number of pairs of orbitals is denoted by the by n). This gives rise to Hückel's rule where aromatic compounds have 4n+2 π electrons which are in a conjugated ring system and are highly stabilised. On the contrary, compounds with 4n π electrons show anti-aromatic behaviour and are highly unstable due to the filling of anti-bonding orbitals.9 O-Xylylene only has 4π electrons in the ring resulting in anti-aromatic instability due to filling of the anti-bonding orbitals. During the course of the reactions, the 6-membered ring of Xylylene becomes a stabilised benzene ring in all 3 pathways as seen in the IRCs. The benzene ring has 6π electrons in a conjugated ring system which obeys Hückel's rule (where n = 1), making it aromatic. Hence the resulting products are more stabilised and the reactions occur favourably.
Gibbs Free Energies
| Xylylene | Sulfur Dioxide | Exo Transition State | Endo Transition State | Chelotropic Transition State | Exo Product | Endo Product | Chelotropic Product | |
|---|---|---|---|---|---|---|---|---|
| Energy/Hartrees | -0.119269 | 0.178119 | 0.092077 | 0.090559 | 0.099062 | 0.021452 | 0.021697 | -0.000018 |
| Energy/kJmol-1 | -313.141 | 467.651 | 241.748 | 237.762 | 260.087 | 56.3222 | 56.9654 | -0.04726 |
Energy Profile Diagram
Diels-Alder reaction with second cis-butadiene fragment
Reaction Scheme
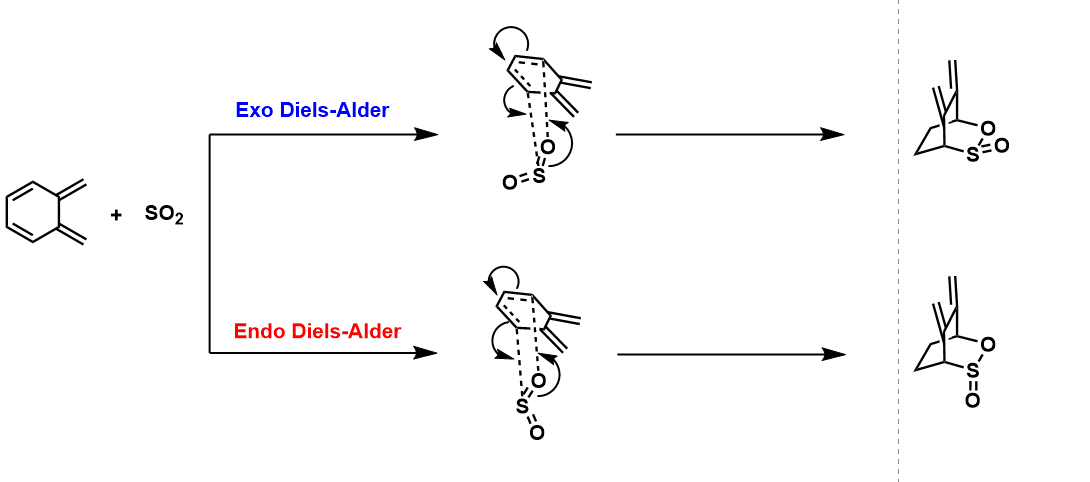
Jmol Files
| Reactants | Transition States | Products | ||||||
|---|---|---|---|---|---|---|---|---|
Reaction Profile Diagram
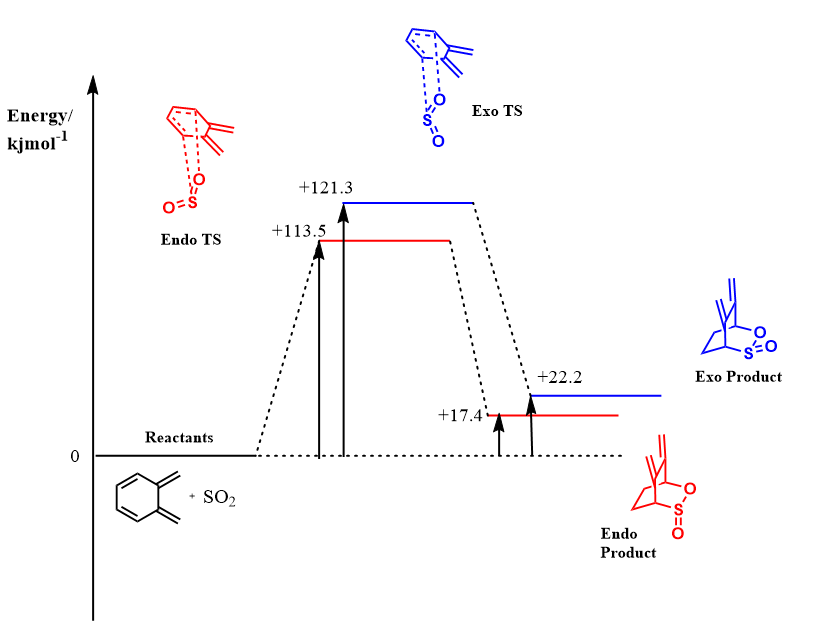
The Endo and Exo Diels-Alder reactions for this cis-butadiene fragment is thermodynamically and kinetically unfavourable. Comparing Figures 6 and 8, the activation energies for both the exo and endo Diels-Alder cycloadditions at this site are much greater than those of the terminal butadiene fragment. Furthermore, the products of the reactions for the non-terminal cis-butadiene fragment are much higher in energy than those for the terminal cis butadiene fragment. The overall reaction for both endo and exo pathways are endothermic in this case. The kinetic and thermodynamic consequences can be rationalised by the greater steric clash present in the transition state and the products when SO2 reacts with the non-terminal cis-butadiene fragment which are absent with the terminal cis-butadiene fragment.
References
9. Aromaticity and the Hückel 4n + 2 Rule. Chemistry Libretexts.
