Rep:Transition States and Reactivity Exercise2 ZWL115
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole
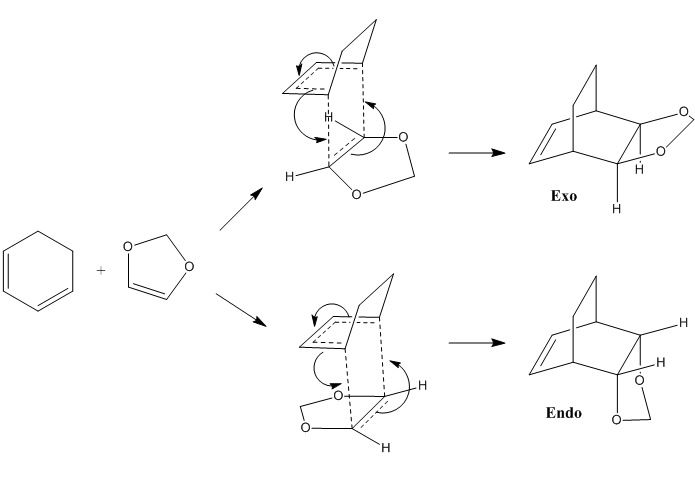
Reaction Scheme
Inverse Demand Diels-Alder Reaction
By running an IRC on the transition states, the structure of the unreacted reactants can be used to run a single point energy calculation. This placed the reactants on the same potential energy surface, allowing the visualisation of the order of the energies of the MOs of the 2 reactants.
| HOMO of Cyclohexadiene | HOMO of 1,3-Dioxole | LUMO of Cyclohexadiene | LUMO of 1,3-Dioxole | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
The MOs in the table are shown in ascending order based on their energies. The HOMO of cyclohexadiene (diene) has a lower energy than the HOMO of 1,3-dioxole (dienophile). This is the opposite from what was observed in Exercise 1 between the reaction of butadiene and ethene. There is a stronger interaction between the HOMO of 1,3-dioxole and the LUMO of cyclohexadiene as they are closer in energy as compared to the LUMO of 1,3-dioxole and the HOMO of cyclohexadiene. Hence this is indicative of a inverse electron demand Diels-Alder reaction . The high energy of the dienophile can be rationalised through the presence of oxygen atoms adjacent to either side of the alkene which have readily available lone pairs of electrons to donate into the π* of the C=C.
Nf710 (talk) 11:00, 9 March 2018 (UTC) Well done for doing this but you should have tabulated the data.
MO diagrams for the formation of the Cyclohexadienediene/1,3-Dioxole transition states
(Fv611 (talk) This is the same diagram twice. You should have discussed, mentioned or shown the differences between the endo and exo conformations in terms of their relative energies.)
| Cyclohexadiene | Endo Transition State | Exo Transition State | 1,3-Dioxole | ||||
|---|---|---|---|---|---|---|---|
 |
 |
||||||
| MO 1 | MO 2 | MO 3 | MO 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endo | ||||||||||||
| Exo |
As mentioned in the previous section, this is an inverse electron demand cycloaddition and hence the HOMO of the dienophile (1,3-Dioxole) is higher in energy than the HOMO of cyclohexadiene. The overall ordering of the energies of the MOs of the transition states are very similar to what was observed in the normal electron demand cycloaddition reaction between butadiene and ethene. The HOMO of the diene interacts with the LUMO of the dienophile to give the bonding MO1 which is lower in energy than the corresponding bonding MO2 between the interaction between the LUMO of the diene and the HOMO of the dienophile. The anti-bonding MO4 from the interaction between the HOMO of the diene and the LUMO of the dienophile is higher in energy than the corresponding MO3 formed from the interaction between the LUMO of the diene and the HOMO of the dienophile. Hence, there is a similar overall destabilisation of the electrons in the transition state as that in the normal electron demand cycloaddition, with weaker bonding and anti-bonding interactions than what would be seen in the corresponding MOs of the products. At the transition state, the overlap of the orbitals are not as strong as those found in the products. Furthermore, it was found that the exo and endo transition states had MOs which were very close in energy. The electrons in the exo transition state occupied electrons which were slightly higher in energy than those in the endo transition state, causing it to be slightly more destabilised.
Gibbs Free Energies
| Cyclohexadiene | 1,3-Dioxole | Exo Transition State | Endo Transition State | Exo Product | Endo Product | |
|---|---|---|---|---|---|---|
| Energy/Hartrees | -233.3243 | -267.0686 | -500.3291 | -500.3321 | -500.4173 | -500.4187 |
| Energy/kJmol-1 | -6.125930 × 105 | -7.011890 × 105 | -1.313614 × 106 | -1.313622 × 106 | -1.313846 × 106 | -1.313849 × 106 |
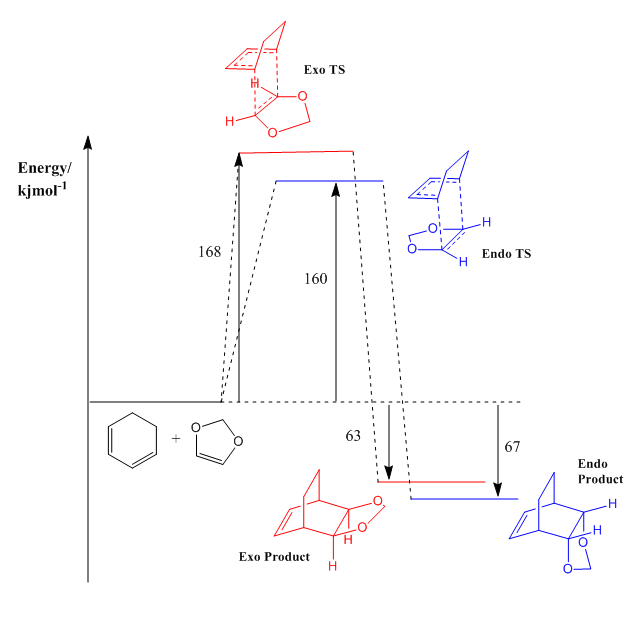
Energy Profile Diagram
Kinetic and Thermodynamic Products
| HOMO of Endo Transition State | HOMO of Exo Transition State | ||||
|---|---|---|---|---|---|
Referring to the Figure 4, the endo product has a lower activation barrier than the exo product. This can be rationalised using the Jmol images of the HOMOs of the endo and exo transition states. In the HOMO of the Endo transition state, there is a good overlap between the p orbitals of the oxygen atoms of dioxole and C=C orbitals of cyclohexadiene. This interaction is absent in the Exo transition state. Furthermore, the greater steric clash of the CH2 units of both the reactants is greater in the Exo transition state than the Endo transition state. Hence, the presence of secondary orbital interactions coupled with the favourable sterics contributes to a lower energy transition state of the endo product. The exo transition state is more unfavourable due to the more significant steric clash present in the transition state and the absence of the secondary orbital interactions. Therefore, the endo product is formed faster than the exo product and the endo product is the kinetic product. The same steric clash present in the transition states is also present in the product, destabilising the exo product. Therefore, the exo product has a higher energy than the endo product. Hence the endo product is also the thermodynamically favoured product.
Nf710 (talk) 11:05, 9 March 2018 (UTC) Excellent section well done. You should have had more decimal places on your energy diagram but I guess you have tabulated the energies so I will let you off. The only place where this could have been improved would have been more detail on the sterics and some theory behind the kenetics and and thermodynamics of the reaction.