Rep:TransitionStates-HYT215-draft
Introduction
- you will have explored advanced techniques in Gaussian, a computational chemistry program, and GaussView, the graphical user interface for Gaussian.
- you should be able to explain what a Transition State and a Potential Energy Surface are.
- you should be able to use chemical intuition to help you to locate stationary points on a Potential Energy Surface.
- you should be able to discuss the roles of sterics and secondary orbital interactions in determining the kinetic and thermodynamic products of a reaction.
- In your introduction, briefly describe what is meant by a minimum and transition state in the context of a potential energy surface. What is the gradient and the curvature at each of these points? (for thought later on, how would a frequency calculation confirm a structure is at either of these points?)
Exercise 1: Reaction of Butadiene with Ethylene
The Diels-Alder reaction of butadiene with ethylene to give cyclohexene is an example of a Diels-Alder reaction. It is a [4+2] cycloaddition between a conjugated diene (butadiene) and dienophile (ethylene), with the reaction scheme given below.
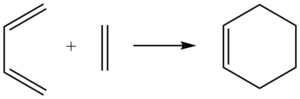
Molecular Orbitals of transition State
Through computational methods done at PM6 level, the HOMOs and LUMOs of the two reactants were obtained as shown below.
| Butadiene | Ethene | ||
|---|---|---|---|
| MO 12
(LUMO) |
MO 7
(LUMO) |
||
| MO 11
(HOMO) |
MO 6
(HOMO) |
||
The interaction of the above four MOs during the transition state gave the four MOs below.
| Transition State | |||
|---|---|---|---|
| MO 16 | MO 17
(HOMO) |
MO 18
(LUMO) |
MO 19 |
By observing the interactions of the orbitals and using the relative energy levels found from the calculations, the MO diagram of the transition state is given below. It is also noted that for the orbitals to interact, they must have the same symmetry labels. If not, the reaction would be forbidden.
The antisymmetric HOMO of butadiene (MO 11) interacts with the antisymmetric LUMO of ethylene (MO 7) to give the two antisymmetric MOs, bonding orbital MO 16 and anti-bonding MO 19 of the cyclohexene transition state. The symmetric LUMO of butadiene (MO 12) interacts with the symmetric HOMO of ethylene (MO 6) to give the two symmetric MOs, bonding orbital MO 17 and anti-bonding MO 18 of the cyclohexene transition state.
Hence, it is concluded that for a symmetric-symmetric or antisymmetric-antisymmetric interaction, the orbital overlap integral is non-zero. However, a symmetric-antisymmetric interaction would be zero.
Bond Lengths
| Jmol | Bond Lengths (unit) | |
|---|---|---|
| Butadiene | ||
| Ethylene | ||
| Transition State | ||
| Cyclohexene |
The two double bonds of the butadiene increase from 1.33 Å to 1.38 Å in the transition state and then to 1.50 Å in the product. These bonds were initially sp2-sp2 C-C double bonds which lengthened to form the sp3-sp2 C-C single bonds.
The single bond of butadiene decreased from 1.47 Å to 1.41 Å in the transition state and then to 1.34 Å in the final product. The bond was initially sp2-sp2 C-C single bond which shortened to form the sp2-sp2 C-C double bond.
The double bond of ethylene increased from 1.33 Å to 1.38 Å in the transition state and then to 1.54 Å in the product. The bond was a sp2-sp2 C-C double bond which lengthened to form a sp3-sp3 C-C single bond.
The bond formation between butadiene and ehtylene was reflected in the decrease in the distance of 2.11 Å during the transition state to 1.54 Å in the product, typical of the sp3-sp3 C-C single bond.
It is noted that the lengths of the C-C single bonds are dependent on the amount of s character. The higher the s character of the orbitals, the shorter the bond. The sp3-sp3 C-C single bonds are longer than the are sp3-sp2 C-C single bonds, which is also longer than the sp2-sp2 C-C single bonds. sp2-sp2 carbons with a higher bond order of two has a shorter length than that of one.
The double bonds of butadiene, ethylene and cyclohexene correspond closely to literature values of alkene of 1.34 Å. The sp3-sp2 C-C single bond of cyclohexene also corresponds to the literature value of 1.50 Å. The sp2-sp2 C-C single bond of butadiene also corresponds to the literature value of 1.47 Å. The sp3-sp3 C-C single bonds of cyclohexene also correspond to the literature value of 1.54 Å. [1] The distance between the two carbons forming the bond of 2.11 Å is smaller than two times the length of the van der Waals radius of carbon (3.4 Å), indicating bond forming or breaking in the transition state.[2]
Reaction Path
The vibration below shows the reaction path at the transition state. As the bond formation between the diene and dienophile took place simultaneously, this bond formation is synchronous.
<Insert reaction path>
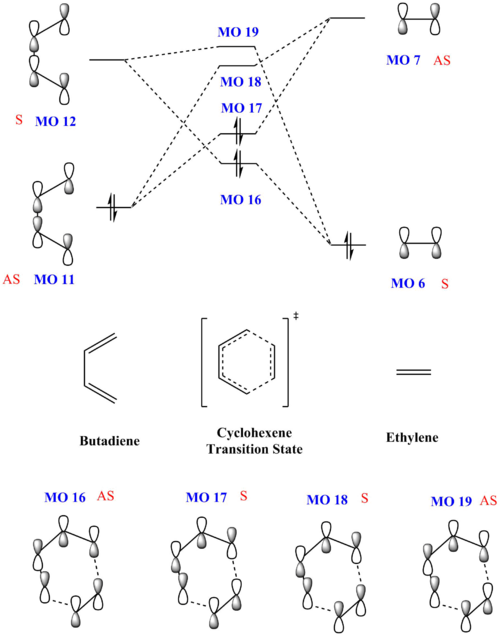
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole
Continuing from the previous exercise, this section explores another Diels-Alder between a cyclohexadiene and 1,3-dioxole where dioxole is the dienophile, with the reaction scheme given below. As the dienophile is now substituted, the direction of approach of dioxole would affect the stereochemistry of the product formed, either an endo- or exo- product.
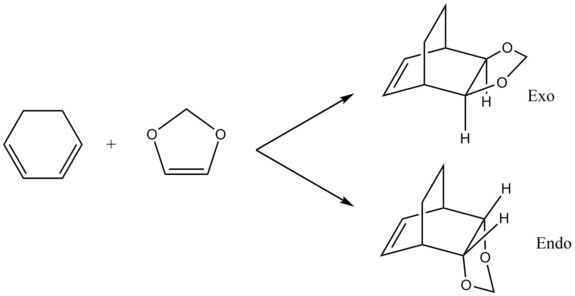
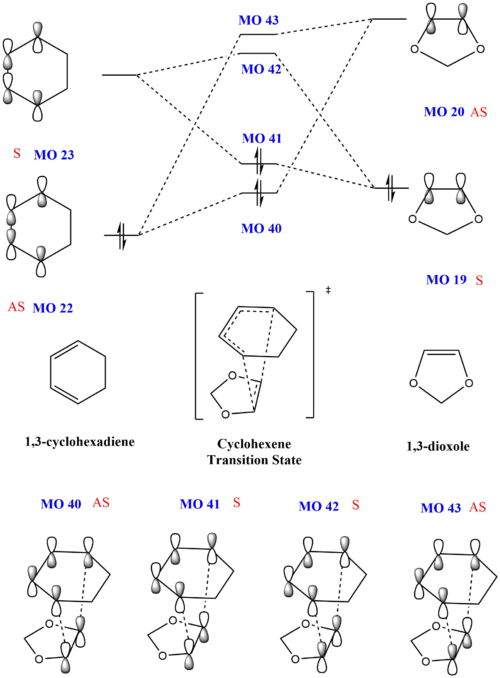
Molecular Orbitals of Transition States
Through computational methods done at B3LYP 6-31G(d) level, the HOMOs and LUMOs of the two reactants were obtained as shown below
| 1,3-cyclohexadiene | 1,3-dioxole | ||
|---|---|---|---|
| MO 23
(LUMO) |
MO 20
(LUMO) |
||
| MO 22
(HOMO) |
MO 19
(HOMO) |
||
The interaction of the above four MOs during the transition state for both endo and exo products gave the four MOs below.
| Transition States | ||||
|---|---|---|---|---|
| MO 40 | MO 41
(HOMO) |
MO 42
(LUMO) |
MO 43 | |
| Exo | ||||
| Endo | ||||
Using the energy levels of MOs derived from the calculations, the following MO diagram was obtained. For a normal Diels-Alder reaction, as shown in exercise 1, the diene is electron rich and has a higher HOMO than the dienophile, which is electron poor. However, in this inverse demand Diels Alder reaction, 1,3-dioxole is an electron rich dienenophile and has a higher HOMO than the cyclohexadiene. This occurs due to the presence of electron rich oxygen atoms adjacent to the C-C double bond on 1,3-dioxole. The electron donating effect of the oxygen atoms lead to 1,3-dioxole having a higher HOMO.
| Exo | Endo |
|---|---|
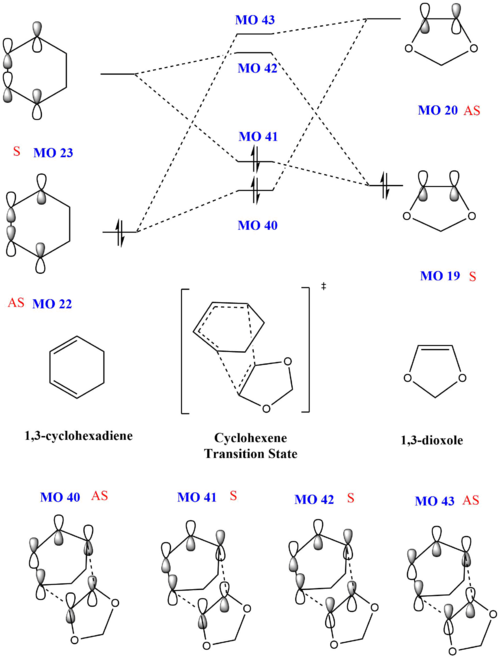
|
|
Energy Calculations
| Energy/ kJmol-1 | |
|---|---|
| 1,3-cyclohexadiene | -6.1259 × 105 |
| 1,3-dioxole | -7.0119 × 105 |
| Sum of Reactants | -1.3138 × 106 |
| Energy/ kJmol-1 | ||||
|---|---|---|---|---|
| Transition State | Product | Reaction Barrier | Reaction Energy | |
| Exo | -1.313614 × 106 | -1.313845 × 106 | 167.71 | -63.744 |
| Endo | -1.313621 × 106 | -1.313849 × 106 | 159.88 | -67.334 |
As the reaction barrier is lower for the endo product, it is kinetically favoured. Due to the reaction energy being lower, the endo product is also thermodynamically favoured.
The HOMO of the transition states were also analysed in greater detail. When the mo cutoff was decrease to 0.01, the interactions for the p-orbitals that were expected from the HOMO (MO 41) of the exo transition state is now clearer as compared to when the isovalue was 0.02 (as seen above). For the HOMO of the endo transition state, there are secondary interactions, further stabilising the transition state, thus lowering its energy. These favourable secondary interactions were not observed for the HOMO of the exo transition state. This is probably why the endo product is kinetically favoured over the exo product.
| Exo | Endo | |
|---|---|---|
| HOMO at isovalue=0.01 | ||
| Schematic |
Exercise 3: Diels-Alder vs Cheletropic
Similar to exercise 2, the competing reactions between o-xylylene and SO2 were examined. Firstly, there are two possible Diels-Alder products, endo and exo. Secondly, there is an additional cheletropic reaction that could take place where the sulfur atom forms a five-membered ring with o-xylylene. These products are shown in the scheme below.

Optimised Transition States
| Diels-Alder (Exo) | Diels-Alder (Exo) | Chelatropic | |
|---|---|---|---|
| Optimised TS | |||
| IRC Cordinates |
Energy Calculations and Reaction Profile
The following calculations of the reactants, transition states and products of both exo and endo Diels Alder and chelatropic products were carried out at PM6 level and tabulated below.
| Energy/ kJmol-1 | |
|---|---|
| o-Xylylene | 469.85 |
| SO2 | -311.42 |
| Sum of Reactants | 158.43 |
| Energy/ kJmol-1 | ||||
|---|---|---|---|---|
| Transition State | Product | Reaction Barrier | Reaction Energy | |
| Exo | 241.75 | 56.330 | 83.318 | -102.10 |
| Endo | 237.77 | 56.976 | 79.339 | -101.46 |
| Cheletropic | 260.08 | -0.0052510 | 101.65 | -158.44 |
From the calculations, the reaction profile was derived and plotted on Microsoft Excel.
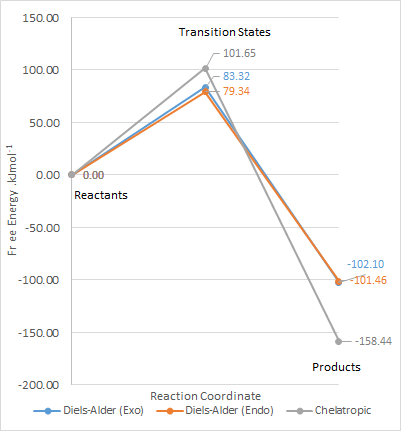
Discuss the different activation energies
IRC
| Diels-Alder (Exo) | Diels-Alder (Exo) | Chelatropic | |
|---|---|---|---|
| Optimised TS | |||
| IRC Cordinates |
From the IRC shown above, the 6-membered ring of o-xylylene initially consisted of 4 C-C single bonds and 2 C-C double bonds. After the reaction, the 6-membered ring gained stability through aromaticity.
Extension
As o-xylylene contains two diene fragments suitable to undergo a Diels-Alder reaction, this section will move on to explore the reaction profile of this reaction relative to exercise 3. The reaction scheme is shown below.

Energy Calculations and Reaction Profile
| Energy/ kJmol-1 | |
|---|---|
| o-Xylylene | 469.85 |
| SO2 | -311.42 |
| Sum of Reactants | 158.43 |
| Energy/ kJmol-1 | ||||
|---|---|---|---|---|
| Transition State | Product | Reaction Barrier | Reaction Energy | |
| Exo | 242.58 | 176.71 | 117.39 | 18.276 |
| Endo | 267.98 | 172.26 | 109.55 | 13.829 |
From the calculations, the reaction profile was derived and plotted on Microsoft Excel.
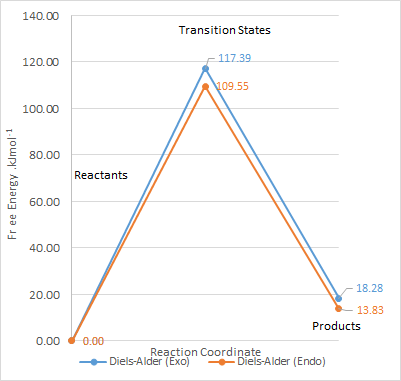
As the activation energy for both the exo and endo product is higher than that of the reaction on the other cis-butadiene fragment site, this site of reaction is less kinetically favourable. The reaction energy is also slightly positive in this case, as compared to negative values in the exercise 3. This shows that the products formed are more unstable than the reactants, and is thermodynamically unfavourable.
