Rep:Title=Mod:eunicemoon316
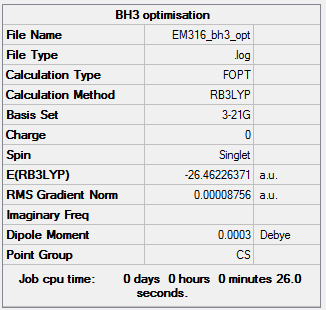
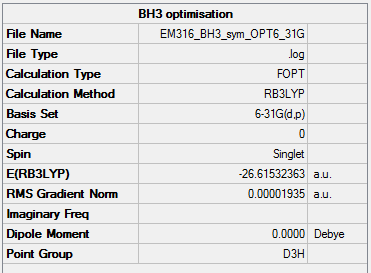
BH3
Method: B3LYP Basis set: 3-21G
Item Value Threshold Converged? Maximum Force 0.000217 0.000450 YES RMS Force 0.000105 0.000300 YES Maximum Displacement 0.000900 0.001800 YES RMS Displacement 0.000441 0.001200 YES
Method: B3LYP Basis set: 6-21G(d,p)
Item Value Threshold Converged? Maximum Force 0.000039 0.000450 YES RMS Force 0.000025 0.000300 YES Maximum Displacement 0.000153 0.001800 YES RMS Displacement 0.000100 0.001200 YES
The frequencies below are the ones recalculated with the right symmetry
Low frequencies --- -19.6657 -16.7777 -16.7646 -0.0054 0.2513 0.6612 Low frequencies --- 1162.8624 1213.0925 1213.0952
Optimised BH |
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 93 | A2 | yes | out-of-plane bend |
| 1213 | 14 | E | very slight | bend |
| 1213 | 14 | E | very slight | bend |
| 2583 | 0 | A1 | no | symmetric stretch |
| 2716 | 126 | E | Yes | asymmetric stretch |
| 2716 | 126 | E | Yes | asymmetric stretch |
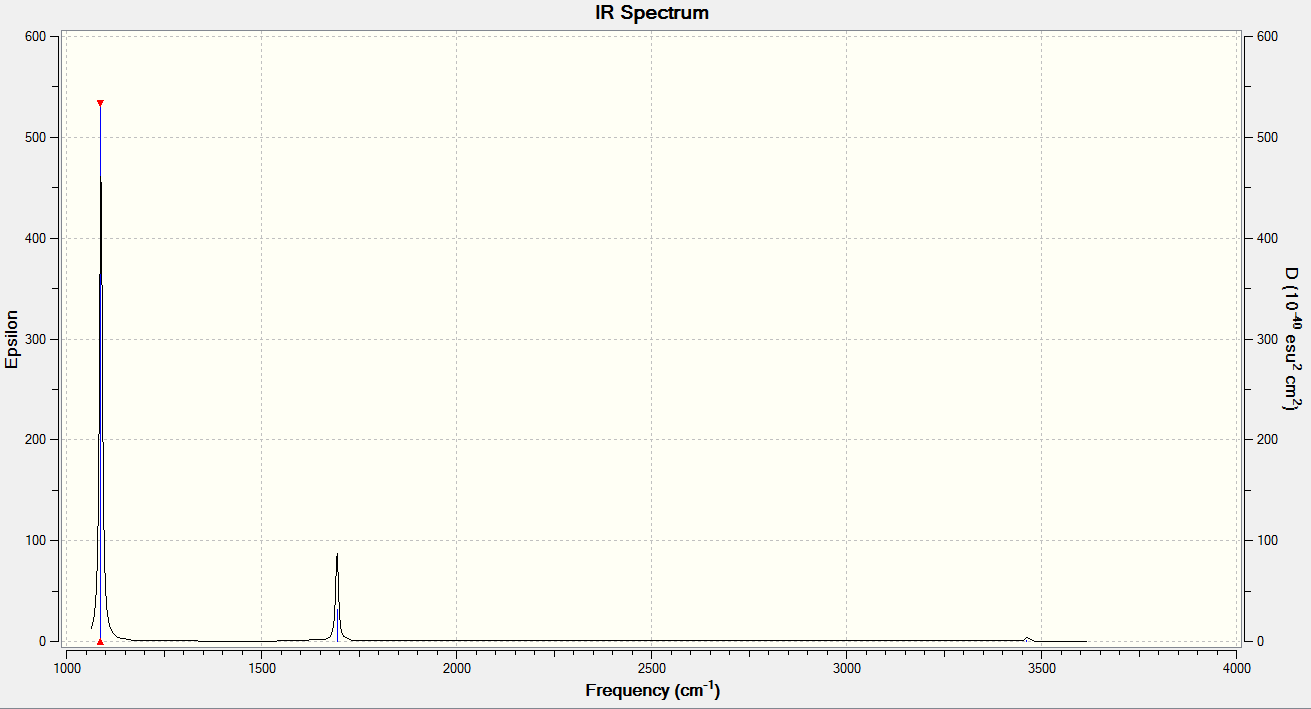
In the frequency table, there are 4 different frequencies observed: 1163, 1213, 2583 and 2716 cm-1 but in the IR spectrum there are only 3 peaks. These peaks correspond to 1163, 1213 and 2716 cm-1. The 2583cm-1 frequency IR inactive as it's a symmetric stretch and there's no change in dipole.
Ng611 (talk) 13:48, 22 May 2018 (BST) It's clear that you've understood that two sets of peaks are degenerate, but I would make the fact that you've observed this more explicit.
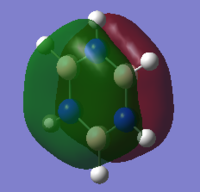
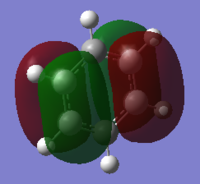
MO diagram of BH3
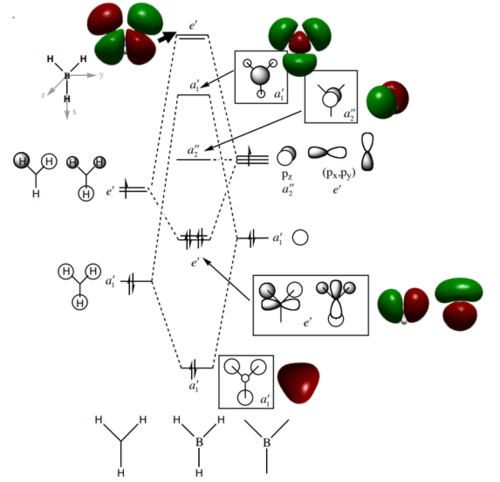
Ng611 (talk) 13:50, 22 May 2018 (BST) Good MO diagram - you're missing a 1e' orbital though (there are 2 in total)
- Are there any significant differences between the real and LCAO MOs?
There isn't mich differenced in the bonding orbital a'1, e' and non-bonding a"2 MOs. But the predicted MO of of antibonding orbital a'1 is differenct from the calculated orbital.
Ng611 (talk) 13:50, 22 May 2018 (BST) Well done for observing that there are differences. How is it different though?
- What does this say about the accuracy and usefulness of qualitative MO theory?
The qualitative MO theory can predict the bonding and non-bonding orbitals accurately but anti-bonding MO may not be accurate.
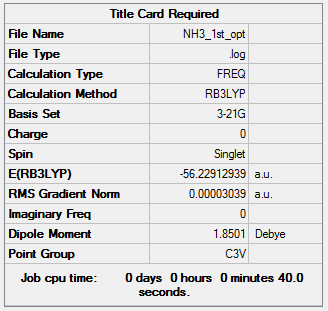
NH3
Method: B3LYP Basis set: 3-21G
Item Value Threshold Converged? Maximum Force 0.000057 0.000450 YES RMS Force 0.000038 0.000300 YES Maximum Displacement 0.000145 0.001800 YES RMS Displacement 0.000096 0.001200 YES Predicted change in Energy=-1.132410D-08
Method: B3LYP Basis set: 6-21G(d,p)
Item Value Threshold Converged? Maximum Force 0.000060 0.000450 YES RMS Force 0.000040 0.000300 YES Maximum Displacement 0.000369 0.001800 YES RMS Displacement 0.000162 0.001200 YES Predicted change in Energy=-2.259205D-08
Optimised BH |
Frequency Analysis
Low frequencies --- -30.2442 -30.2441 -27.9027 -0.0013 0.0008 0.0030 Low frequencies --- 1088.3853 1693.7756 1693.7756
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1088 | 146 | A1 | yes | out-of-plane bend |
| 1694 | 14 | E | very slight | bend |
| 1694 | 14 | E | very slight | bend |
| 3462 | 1 | A1 | no | symmetric stretch |
| 3591 | 0 | E | Yes | asymmetric stretch |
| 3591 | 0 | E | Yes | asymmetric stretch |
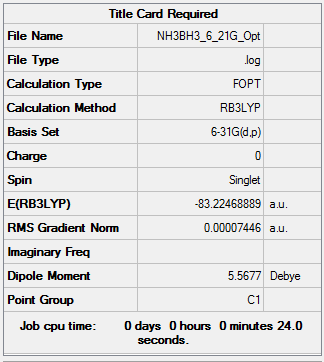
NH3BH3
Method: B3LYP Basis set: 6-21G(d,p)
Optimised BH |
Item Value Threshold Converged? Maximum Force 0.000131 0.000450 YES RMS Force 0.000037 0.000300 YES Maximum Displacement 0.000654 0.001800 YES RMS Displacement 0.000179 0.001200 YES Predicted change in Energy=-1.115346D-07
Vibrational spectrum
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 264 | 0 | A2 | no | N-B rotation |
| 634 | 14 | E | slight | N-B stretching |
| 639 | 4 | E | very slight | N-B twsting |
| 639 | 4 | A1 | very slight | N-B rocking |
| 1069 | 41 | E | Yes | N-B twsting |
| 1069 | 41 | E | Yes | N-B rocking |
| 1196 | 108 | E | Yes | B-H out-of-plane bend |
| 1204 | 3 | E | very slight | N-H bend |
| 1204 | 3 | E | very slight | N-H bend |
| 1330 | 113 | E | Yes | N-H out-of-plane bend |
| 1676 | 28 | E | Yes | N-H bend |
| 1676 | 28 | E | Yes | N-H bend |
| 2472 | 67 | E | Yes | B-H symmetric stretch |
| 2532 | 231 | E | Yes | B-H asymmetric stretch |
| 2532 | 231 | E | Yes | B-H asymmetric stretch |
| 3464 | 2 | E | very slight | N-H symmetric stretch |
| 3580 | 28 | E | Yes | N-H asymmetric stretch |
| 3580 | 28 | E | Yes | N-H asymmetric stretch |
Energy Calculation
E(NH3)= -26.46226 a.u. E(BH3)= -56.55776 a.u. E(NH3BH3)= -83.22468 a.u.
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] =-83.22468 -(-26.46226 + -56.55776) =-0.20466 a.u. =-537.33483 kJ/mol
Ng611 (talk) 13:55, 22 May 2018 (BST) You've got your BH3 and NH3 values the wrong way around here (According to your summary tables, NH3, not BH3 has a self-energy of -56.55777 hartrees). You've also used the wrong energy for BH3 (which you've labelled as NH3) - the value you use is the one from your B3LYP/3-21G optimisation rather than the B3LYP/6-31G optimisation. Remember that you cannot compare values obtained using different basis sets. You have performed the calculation correctly though!
The bond dissociation energy has a negative value as it's an exothermic reaction. The calculated value is negative which fits in with the theory. Also the bond strength varies between 3-1000 kl/mol therefore -537 kJ/mol is a sensible value for a bond dissociation energy.
- Based on your energy calculation is the B-N dative bond weak, medium or strong? What comparison have you made to come to this conclusion?
The energy of B-N bond is medium. One of the strong bonds NΞN dissociation energy is 945 kJ/mol and C-O bond's dissociation energy is 1077 kJ/mol. Weak bonds such as Br-Br has a bond dissociation energy value of 192 kJ/mol.[2] B-N bond has a dissociation energy value in between the strong and weak bond dissociation energy, therefore, B-N bond energy is medium.
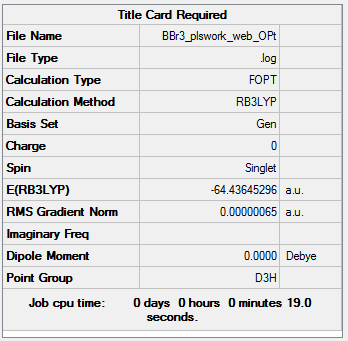
BBr3
Method: B3LYP Basis set: 6-21G GEN
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000006 0.001800 YES RMS Displacement 0.000004 0.001200 YES Predicted change in Energy=-1.206088D-11 Optimization completed.
Low frequencies --- -0.0116 -0.0065 -0.0004 49.9506 49.9506 50.0315 Low frequencies --- 144.7606 144.7640 215.6181
Optimised BH |
Aromaticity
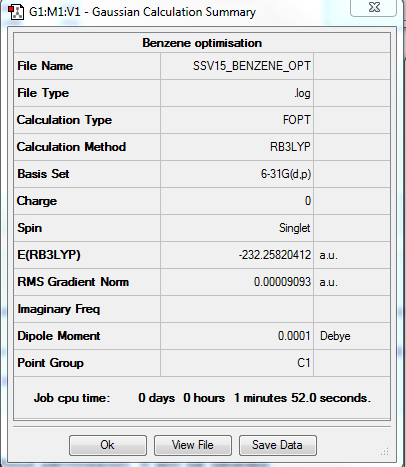
Benzene
Method: B3LYP Basis set: 6-21G(d,p)
Optimised BH |
Item Value Threshold Converged? Maximum Force 0.000049 0.000450 YES RMS Force 0.000021 0.000300 YES Maximum Displacement 0.000068 0.001800 YES RMS Displacement 0.000030 0.001200 YES Predicted change in Energy=-8.796913D-09
Frequency Analysis
Low frequencies --- -11.2053 -7.2445 -7.2445 -0.0055 -0.0055 -0.0007
Low frequencies --- 414.4985 414.4985 621.0620
Diagonal vibrational polarizability:
0.2795691 0.2795903 4.1346737
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
E2U E2U E2G
Frequencies -- 414.4985 414.4985 621.0620
Red. masses -- 2.9462 2.9462 6.0756
Frc consts -- 0.2982 0.2982 1.3807
IR Inten -- 0.0000 0.0000 0.0000
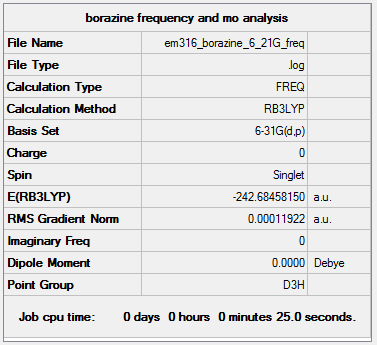
Borazine
Method: B3LYP Basis set: 6-21G(d,p)
Optimised BH |
Item Value Threshold Converged? Maximum Force 0.000290 0.000450 YES RMS Force 0.000119 0.000300 YES Maximum Displacement 0.000581 0.001800 YES RMS Displacement 0.000278 0.001200 YES Predicted change in Energy=-4.469792D-07
Frequency Analysis
Low frequencies --- -14.2714 -13.9795 -10.8868 -0.0110 0.0326 0.0561
Low frequencies --- 289.1178 289.1292 404.2716
Diagonal vibrational polarizability:
7.3588310 7.3578646 14.1611762
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
E" E" A2"
Frequencies -- 289.1170 289.1284 404.2716
Red. masses -- 2.9298 2.9298 1.9279
Frc consts -- 0.1443 0.1443 0.1856
IR Inten -- 0.0000 0.0000 23.7973
Benzene and Borazine comparison
Charge distribution
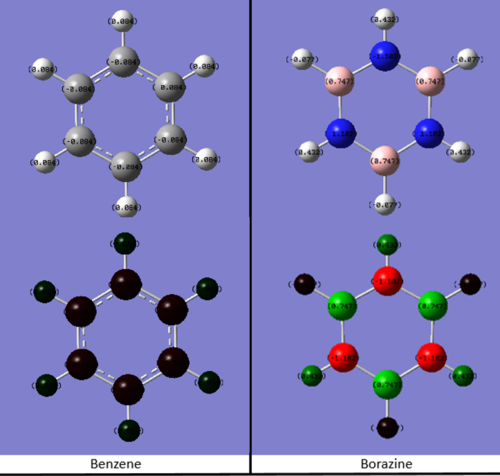
The charge distribution on benzene is: C= -0.084 eV, H = 0084. eV For the Borazine: B= 0.747 eV, N= -1.102 eV, H(N-H)= 0.432 eV, H(B-H)= -0.077 eV
For benzene all the carbon molecule has a same charge; -0.084 eV. And the charge is negative as carbon is more electronegative than hydrogen thus there's more electron density on carbon than the hydrogen.
The charge differences in borazine can be explained through the same principle: nitrogen is more electronegative than both boron and hydrogen thus it has the most negative charge. Therefore the charge on hydrogen of N-H bond is slightly more positive than the hydrogen in B-H bond: the electron density on hydrogen is pulled towards the electronegative nitrogen. On the other hand, boron is less electronegative than hydrogen so B-H hydrogen has slightly negative charge.
Ng611 (talk) 14:00, 22 May 2018 (BST) Good discussion of the effects of electronegativity on the overall charge distribution. What do the partial charges sum to, and is there any difference in partial charge for atoms related by symmetry?
Molecular Orbitals
Ng611 (talk) 14:02, 22 May 2018 (BST) Good description of the MOs. I'd add some more discussion about why the differences are there to strengthen this discussion further. You've done it a bit for MO20, but I'd expand on this further and also repeat this discussion for the other two MOs.
Aromaticity
MO20 for both benzene and borazine, delocalised electron density above and below the nodal plane can be observed. This relates to the pi-electron delocalisation which is a common concept of aromatic molecules.
On the other hand, just relying on the overlap of Pz AOs to account for aromaticity is not suitable. The key concept of aromaticity is the aromatic stability which is result of pi-delocalisation. However, not only pi-electron structure contributes to the stability but sigma electron structure as well. [3]
Also there are other factors which needs to be considered for a molecule to be aromatic: The molecule must be cyclic. The ring needs to be planar. Each atom in the ring must be sp2-hybridised. The number of pi-electrons must obey the Huckle's rule(4n+2).[4]
Ng611 (talk) 14:05, 22 May 2018 (BST) What about benzene at low temperatures (~ 20K). It is definitely not planar then - will it still be aromatic?
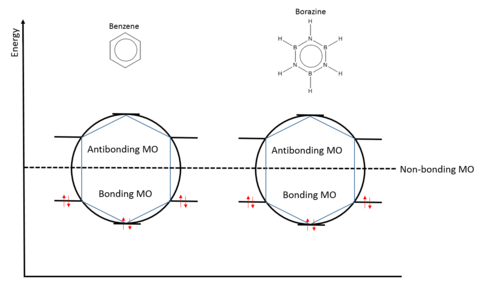
In the frost circle diagram, both benzene and borazine have 6 electrons occupying all three pi MO but anti-bonding MOs are not occupied. Fully occupied bonding orbitals provide 'full shell' of electrons, which creates a similar effect to a full shell of electrons in noble gasses, thus stabilising the molecules.[5] Therefore, there are many factors in aromatic stability than just a delocalisation of pi electrons.
Ng611 (talk) 14:05, 22 May 2018 (BST) You've got the basic points of aromaticity down certainly, well done. Some additional detailed discussion regarding how the conceptual picture of aromaticity has evolved would improve this section further - as would a discussion of how aromaticity con be observed experimentally.
Reference
- ↑ MO course handout, Trisha Hunt, 2017
- ↑ https://en.wikipedia.org/wiki/Bond-dissociation_energy
- ↑ Application of AIM Parameters at Ring Critical Points for Estimation ofp-Electron Delocalization in Six-Membered Aromatic andQuasi-Aromatic Rings,Palusick,M ,Krygowski,T ,
- ↑ https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry_Textbook_Maps/Map%3A_Organic_Chemistry_with_a_Biological_Emphasis_(Soderberg)/Chapter_02%3A_Introduction_to_organic_structure_and_bonding_II/2.2%3A_Molecular_orbital_theory%3A_conjugation_and_aromaticity
- ↑ http://www.chemgapedia.de/vsengine/vlu/vsc/en/ch/12/oc/vlu_organik/aromaten/aromaten/aromaten_gesamt.vlu/Page/vsc/en/ch/12/oc/aromaten/aromaten/frost/frost.vscml.html
Ng611 (talk) 14:09, 22 May 2018 (BST) Overall a good report in many places. Some additional detail in your discussions - particularly in the second section would have improved the report significantly. Remember also to 'State the Obvious' to demonstrate to us that you've grasped the subject matter. However, your MO analysis was good, the calculations were performed well, and you did well to include the key results.