Rep:Title=Mod:JXC2209
NH3 Molecule
test molecule |
OPTIMISATION
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
Final Energy E(RB3LYP) = -56.49570525 au
RMS Gradient = 0.04919263 au
Point Group = C3v
N-H Bond Distance = 1.21312 A
H-N-H Bond Angle = 107.064 degrees
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
The optimisation file is linked to here
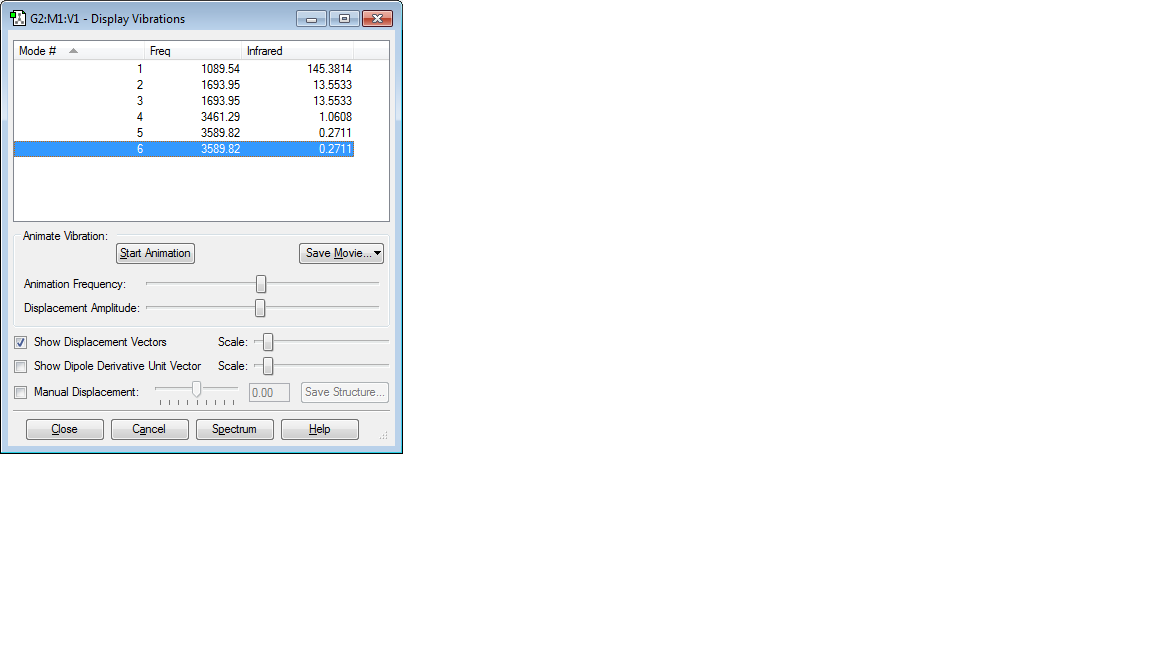
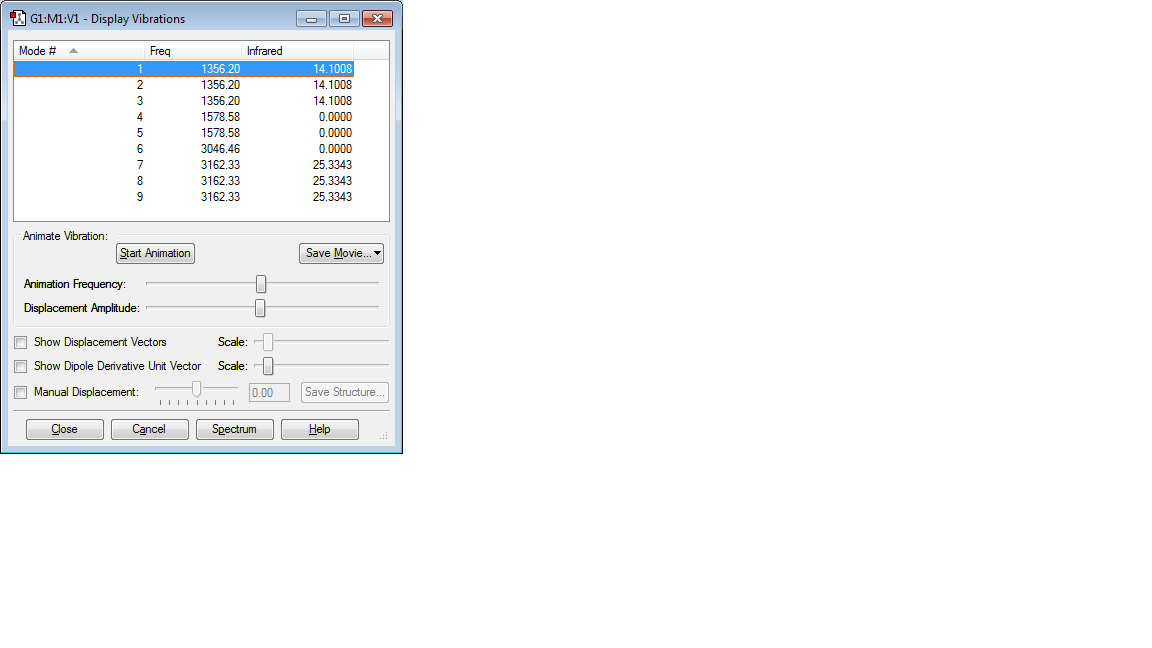
VIBRATIONS
Number of Modes Expected = 3(4)-6 = 6
Degenerate Modes = 2 and 3, 5 and 6
Bending Vibrations = 1,2 and 3
Bond Stretch Vibrations = 4,5 and 6
Highly Symmetric Mode = 6
Umbrella Mode = 1
No of Bands expected in an Experimental Spectrum of gaseous NH3 = 4
Since N is more electronegative than H, I expect N to have a negative charge while the 3H have a positive charge.
Charge on N = -1.125
Charge on H = 0.375
N2 Molecule
test molecule |
OPTIMISATION
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
Final Energy E(RB3LYP) = -109.52412868 au
RMS Gradient = 0.02473091 au
Point Group = D§h
N-N Bond Distance = 1.10550 A
N-N Bond Angle = 180 degrees
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
The optimisation file is linked to here
VIBRATIONS
Number of Modes Expected = 3(2)-5 = 1
Degenerate Modes = None
Bending Vibrations = None
Bond Stretch Vibrations = 1
No of Bands expected in an Experimental Spectrum of gaseous N2 = 1
H2 Molecule
test molecule |
OPTIMISATION
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
Final Energy E(RB3LYP) = -1.151265854 au
RMS Gradient = 0.09719500 au
Point Group = D§h
H-H Bond Distance = 0.60000 A
H-H Bond Angle = 180 degrees
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
The optimisation file is linked to here
VIBRATIONS
Number of Modes Expected = 3(2)-5 = 1
Degenerate Modes = None
Bending Vibrations = None
Bond Stretch Vibrations = 1
No of Bands expected in an Experimental Spectrum of gaseous H2 = 1
Energy of Reaction N2 + 3H2 -> 2NH3
E(NH3)= -56.49570525 au
2*E(NH3)=-112.9914105 au
E(N2)= -109.52412868 au
E(H2)= -1.151265854 au
3*E(H2)= -3.453797562 au
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -112.9914105 au + 109.52412868 au + 3.453797562 au
= -0.013484258 au
= -35.40291938 kJ/mol
Since ΔE>0, the reaction is exothermic and the ammonia product is more stable.
My results show that the gaseous products are higher in energy than the ammonia product, which logically makes sense. Comparing my results with literature, the experimental value is approximately -92.4 kJ/mol which is much more exothermic than the value obtained in my calculations.
Choice of Molecule :CH4
test molecule |
OPTIMISATION
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
Final Energy E(RB3LYP) = -40.52275298 au
RMS Gradient = 0.00803796 au
Point Group = Td
C-H Bond Distance = 1.07000 A
H-C-H Bond Angle = 109.471 degrees
Item Value Threshold Converged?
Maximum Force 0.000063 0.000450 YES
RMS Force 0.000034 0.000300 YES
Maximum Displacement 0.000179 0.001800 YES
RMS Displacement 0.000095 0.001200 YES
The optimisation file is linked to here
VIBRATIONS
Number of Modes Expected = 3(5)-6 = 9
Degenerate Modes = 1,2 and 3; 4 and 5; 7,8 and 9
Bending Vibrations = 1,2,3,4 and 5
Bond Stretch Vibrations = 6,7,8 and 9
Highly Symmetric Mode = 6
No of Bands expected in an Experimental Spectrum of gaseous NH3 = 4
Since C is more electronegative than H, I expect C to have a negative charge while the 4H have a positive charge.
Charge on N = -0.930
Charge on H = 0.233
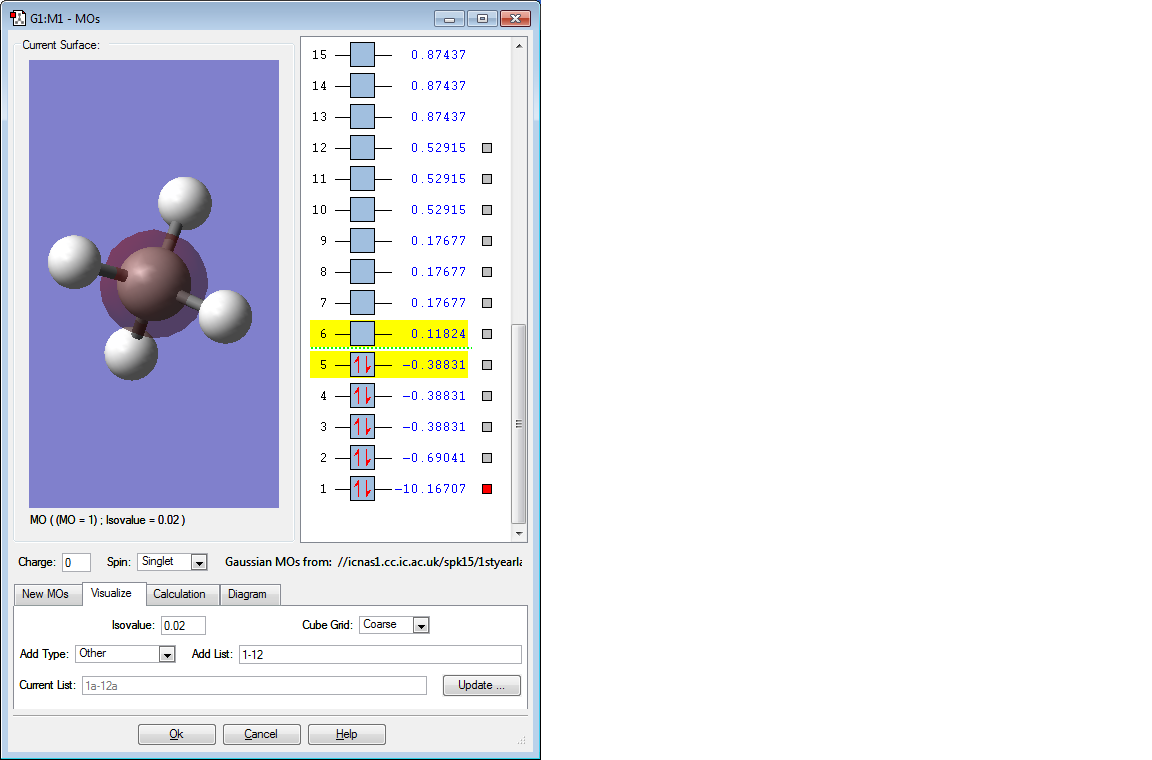
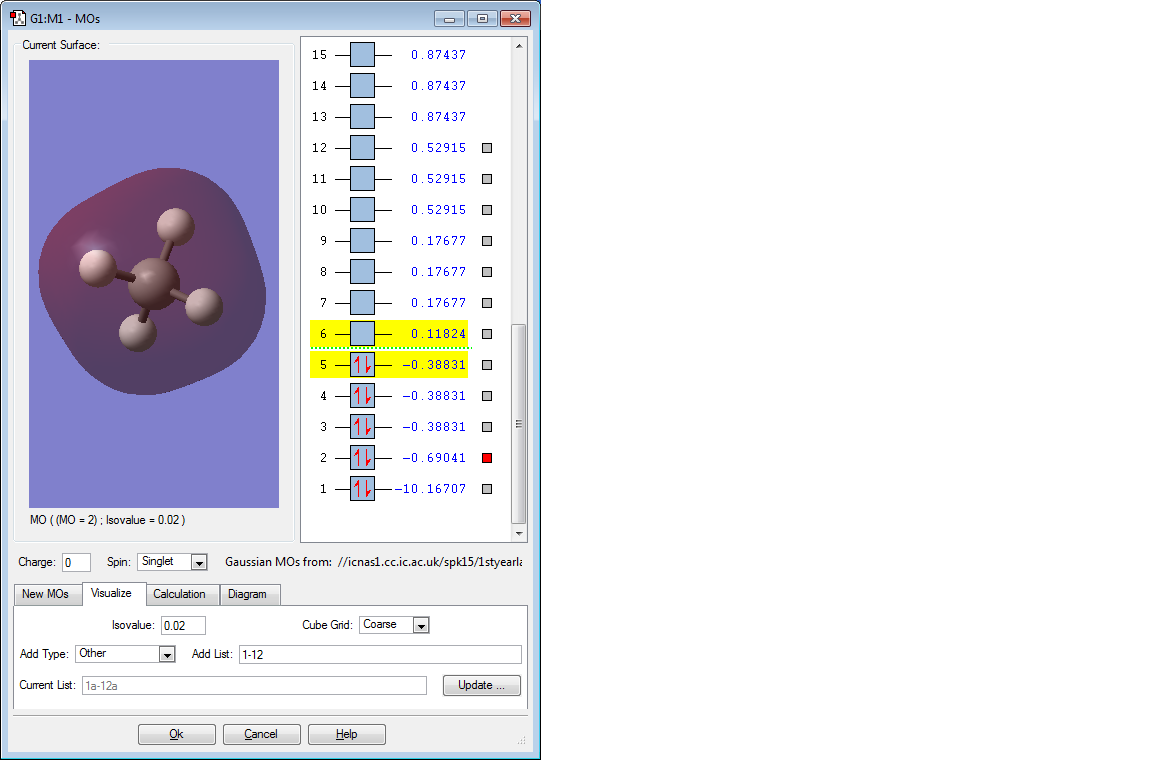
MOLECULAR ORBITALS
This MO is the lowest energy MO. It is extremely deep in energy, -10.16707 au, because it is formed by the AO of the two 1s electrons in C. Clearly this MO only covers the C atom and there is little overlap with the neighbouring H atoms since the electrons are held tightly to the C nuclei.
In the promoted state of C, there is one electron in the 2s orbital, which is at lower energy, and 3 in the 2p orbitals. Thus, the above MO is a bonding MO formed by the overlap of the 1s electron of 1 H atom and the 2s electron of the C atom. It is a much stronger and extensive interaction, as seen by how the MO is displayed as a sphere around the entire CH4 molecule.
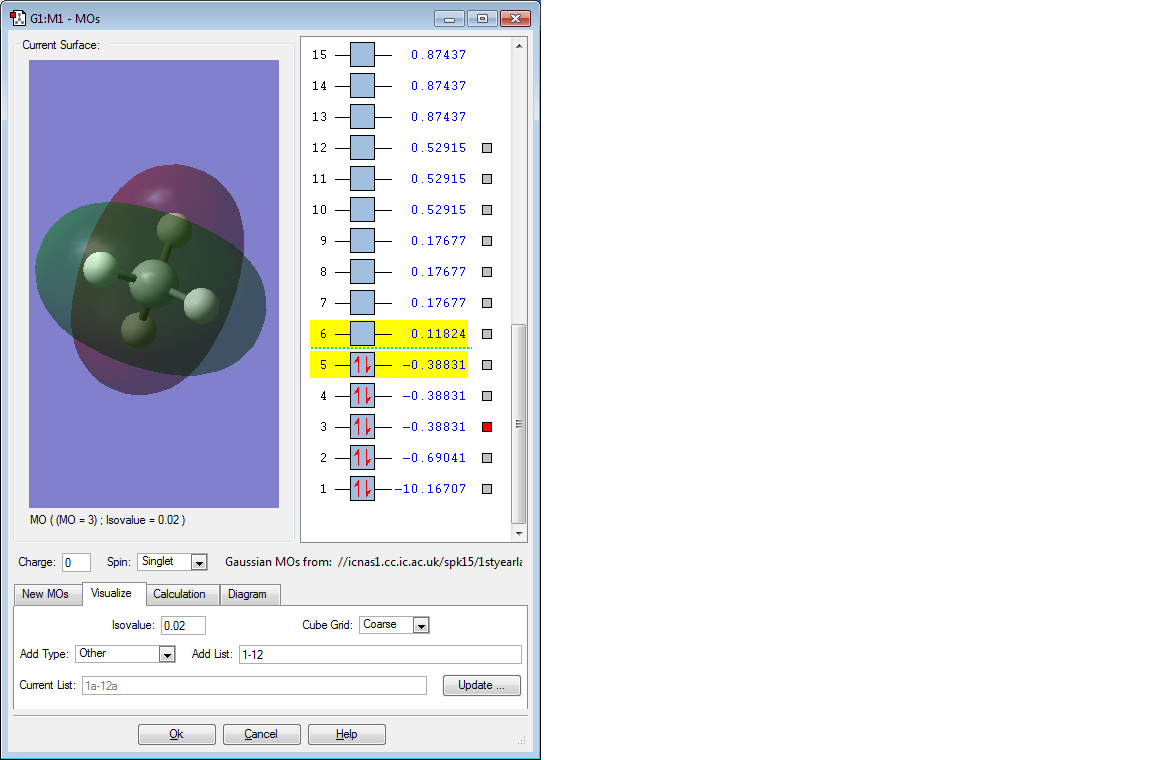
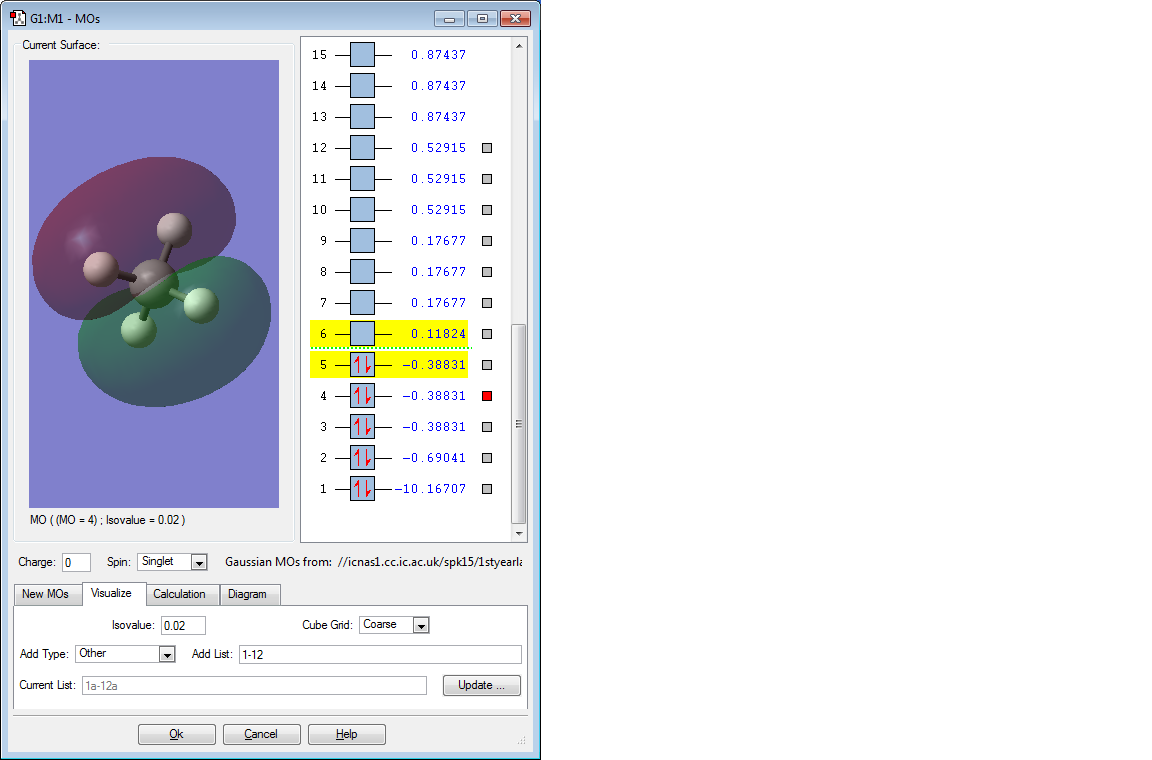
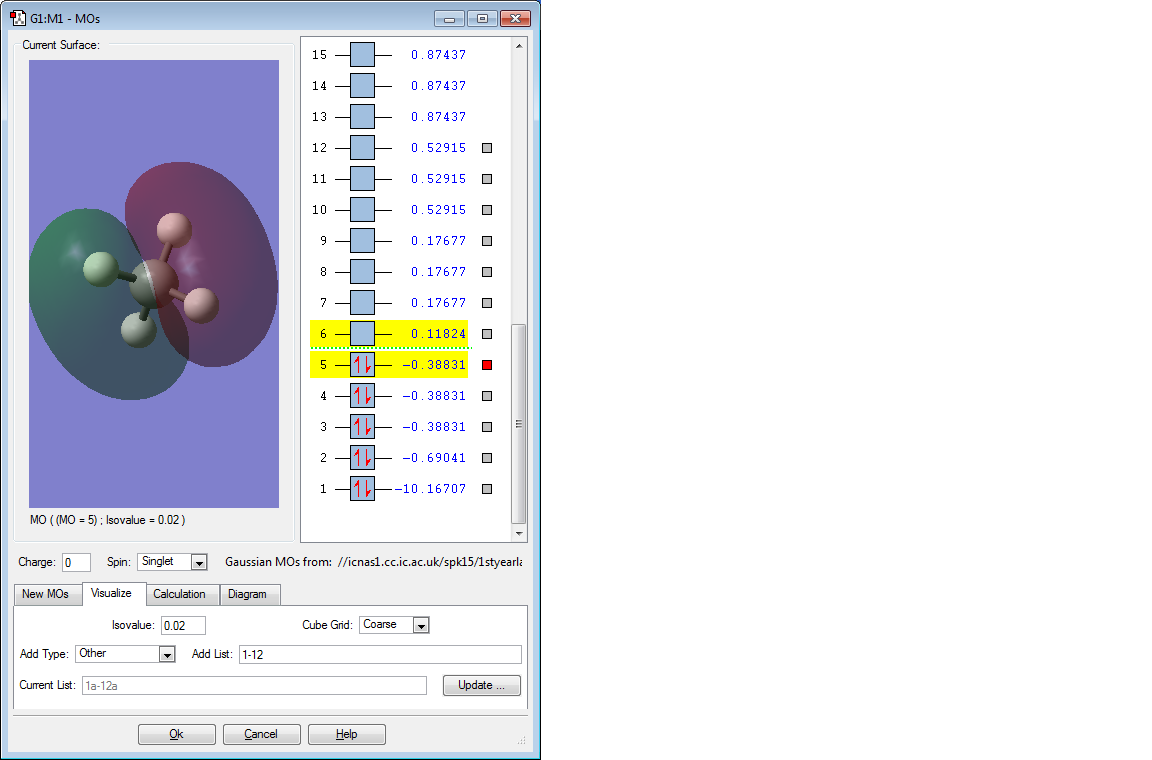
The above 3 bonding MOs are all degenerate. They are formed by the overlap of the 3 electrons in the 2p orbital of C and the remaining 3 1s orbitals of H. They are all also the HOMO energy level and are pi bonding MOs.
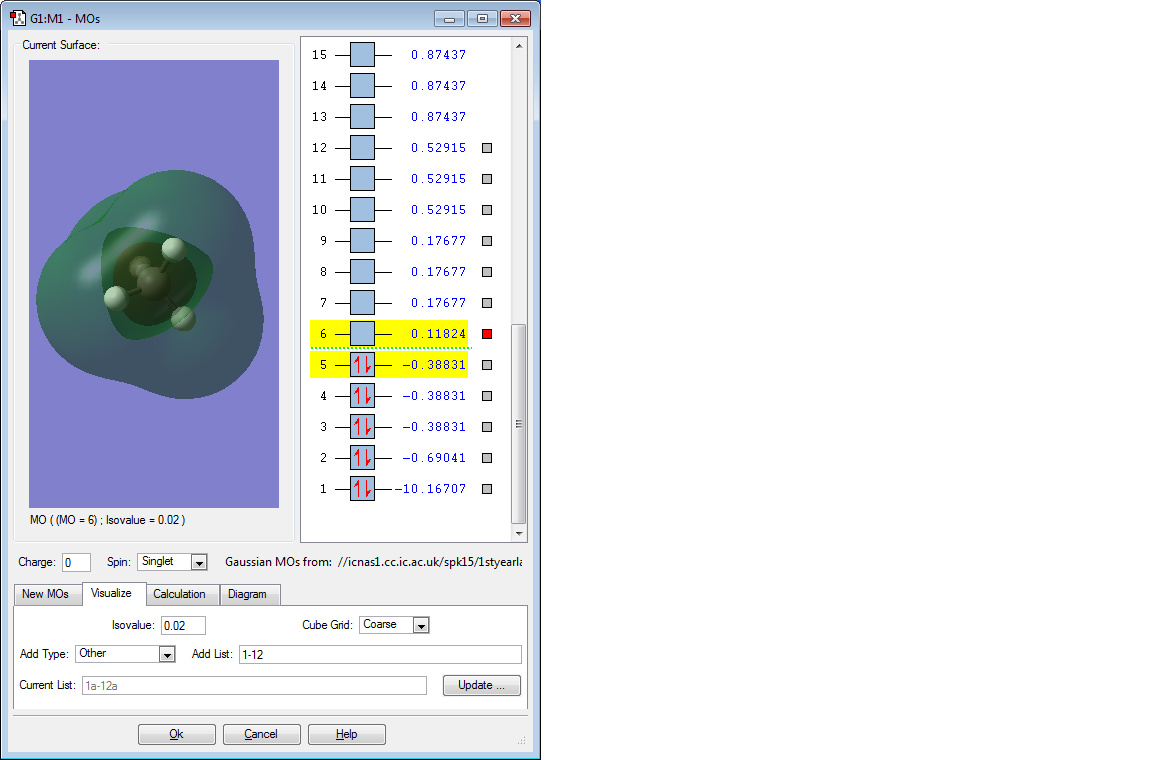
The above MO is the antibonding MO formed by the out of phase overlap between the lone electron in the 2s orbital of the C atom and 1s electron of the H atom. This orbital is extremely high in energy such that is has a positive value, +0.11824 au.