Rep:Title=Mod:19SAXON
NH3 Molecule
Calculation Method:
RB3LYP
Basis Set:
6-31G(d,p)
Final Energy, E(RB3LYP) (au):
-56.55776873
RMS Gradient (au):
0.00000485
Point Group:
C3V
N-H Bond Length (au):
1.01798
H-N-H Bond Angle (deg):
105.741
Item Table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Optimisation file link
NH3 |
Vibrations
Questions:
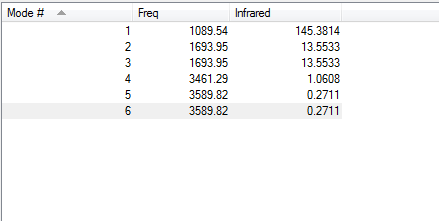
-How many modes do you expect from the 3N-6 rule?
6 modes would be expected.
-Which modes are degenerate?
Modes 2,3 and 5, 6 are degenerate.
-Which modes are "bending" vibrations and which are "bond stretch" vibrations?
Bending: 1, 2, 3 Stretching: 4, 5, 6
-Which mode is highly symmetric? Mode 4 is highly symmetric.
-Which mode is known as the "umbrella" mode?
Mode 1 is the "umbrella" mode.
-How many bands would you expect to see in an experimental spectrum of gaseous ammonia?
You would expect to see 2 bands in the spectrum because mode 4 is highly symmetric and there is no change in dipole moment. Whilst there is a change in dipole moment for modes 5 and 6 the intensities are so low that they cannot be seen in a spectrum.
Charge Analysis
N atom charge= -1.125
H atom charge= +0.375
This is what was expected considering the high electronegativity of nitrogen compared to hydrogen.
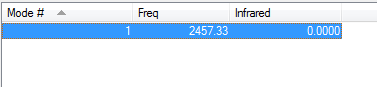
N2 Molecule
Calculation Method:
RB3LYP
Basis Set:
6-31G(d,p)
Final Energy, E(RB3LYP) (au):
-109.52412868
RMS Gradient (au):
0.00000060
Point Group:
D*h
N-N Bond Length (au):
1.10550
Item Table
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Vibrations
Optimisation file link
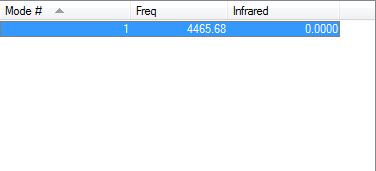
H2 Molecule
Calculation Method:
RB3LYP
Basis Set:
6-31G(d,p)
Final Energy, E(RB3LYP) (au):
-1.17853936
RMS Gradient (au):
0.00000017
Point Group:
D*h
H-H Bond Length (au):
0.74279
Item Table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Vibrations
Optimisation file link
Reaction Energies
The reaction energy (ΔE) is being calculated for the reaction: N2 + 3H2 -> 2NH3
(au)
E(NH3)=-56.55776873
2*E(NH3)=-113.11553746
E(N2)=-109.52412868
E(H2)=-1.17853936
3*E(H2)=-3.53561808
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=-0.0557907
ΔE(KJ/mol)=-146.48
Compared to the literature value for Std enthalpy change of formation= −45.92 kJ/mol (for one NH3), =-91.84KJ/mol. (From: Cox, J.D.; Wagman, D.D.; Medvedev, V.A., CODATA Key Values for Thermodynamics, Hemisphere Publishing Corp., New York, 1984, 1)
The differences between the calculated value and the literature value stem from the limitations of the calculation. The calculation doesn't take into account thermodynamic considerations or rotational or vibrational energies.
BH4- Molecule
Calculation Method:
RB3LYP
Basis Set:
6-31G(d,p)
Final Energy, E(RB3LYP) (au):
-27.24992701
RMS Gradient (au):
0.00000671
Point Group:
TD
B-H Bond Length (au):
1.23933
H-B-H Bond Angle (deg):
109.471
Item Table
Item Value Threshold Converged? Maximum Force 0.000013 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000065 0.001800 YES RMS Displacement 0.000035 0.001200 YES
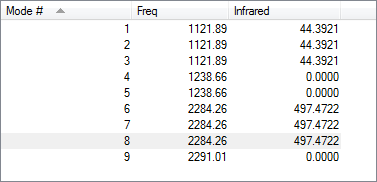
Vibrations
Two peaks would be expected in the experimental gaseous spectrum for BH4-. This is because of the degeneracy of modes 1-3 and 6-8. All other modes are inactive as due to their highly symmetric nature leading to no change in dipole moment.
Charge Analysis
N atom charge= -0.615
H atom charge= -0.096
This is what was expected considering the overall charge of the molecule.
Optimisation file link
BH4- |
Molecular Orbitals
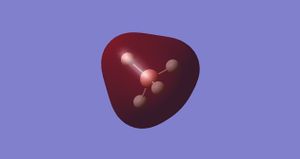
Molecular Orbital 1-
 This is the non-bonding orbital from the very deep in energy 1s AO from boron. It is so low in energy that there is little/no interaction with the 1s orbitals on the hydrogen.
This is the non-bonding orbital from the very deep in energy 1s AO from boron. It is so low in energy that there is little/no interaction with the 1s orbitals on the hydrogen.
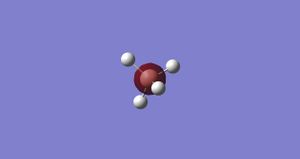
Molecular Orbital 2-
 This is the bonding orbital from the combination of the 1s orbitals from the hydrogen's and the 2s orbital on the boron.
This is the bonding orbital from the combination of the 1s orbitals from the hydrogen's and the 2s orbital on the boron.
These are the degenerate bonding orbitals which arise form the combinations of the filled p orbitals and the 1s orbital on the hydrogen's. The are the highest occupied molecular orbitals and are quite high in energy for bonding orbitals.
Joseph O'Connell-Danes