Rep:TSR:ZX2015Y3C
Introduction
The minimum state is the reactant or product whichever has the lower energy and the transition state is where the system reaches its highest energy. Both of these states have gradient of zero. The minimum state has positive curvature while the transition state has negative curvature.
At the minimum state, all frequencies calculated should be positive. At transition state, all frequencies, apart from the vibration responsible for the reaction (negative), should be positive.
22:33, 30 November 2017 (UTC) This is correct for 1 dimension but our PES has 3N-6 dimensions (these are also the degrees of freedom)
Exercise 1: Reaction of Butadiene with Ethylene
(Fv611 (talk) There was almost no discussion, even though you clearly performed the right calculations. As a third year, you are expected to critically assess your results, not just report them.)
MO diagram
At the transition state the system has the highest energy in the reaction, hence the energy levels of all MOs formed shift upward.
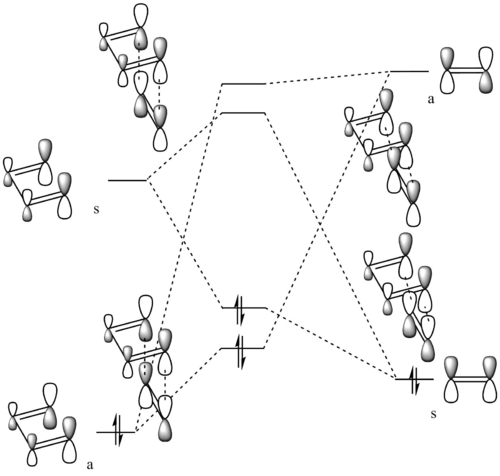
- The combination of MOs from reactants gives MOs with the same symmetry in the transition state.
(Fv611 (talk) Your MO is good, and I especially appreciate the reporting of the energies of the TS MOs, but you should have labelled your FMOs and TS MOs on the diagram to correlate them with those you present in the table.)
Visualising MOs
Symmetry requirement for the reaction can be concluded as only molecular orbitals with the same symmetry label can combine to form new molecular orbital.
Orbital overlap integral can be surmised as below:
| Symmetric | Asymmetric | |
|---|---|---|
| Symmetric | Non-zero(allowed) | Zero(forbidden) |
| Asymmetric | Zero(forbidden) | Non-Zero(allowed) |
Reactants
| HOMO | LUMO | |||||
|---|---|---|---|---|---|---|
| Butadiene | ||||||
| Ethylene |
Transition State
| Asymmetric Antibonding | Symmetric Antibonding | Symmetric Bonding | Asymmetric Bonding | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Energy | 0.03067au | 0.01732au | -0.32534au | -0.32756au | ||||||||
| MOs |
Bond Length
Butadiene
Ethylene
Transition State
Product
Summery
| Bond Lengths/nm | Reactant | Transition State | Product | Trend |
|---|---|---|---|---|
| Dienophile | 0.133 | 0.138 | 0.153 | Increase(Weaken) |
| Diene(Terminal) | 0.133 | 0.138 | 0.150 | Increase(Weaken) |
| Diene(Bridging) | 0.147 | 0.141 | 0.134 | Decrease(Strengthen) |
| New formed | - | 0.211 | 0.154 | - |
Typical sp3-sp3 C-C bond has length of 0.154 nm; sp2-sp3 C-C bond has length of 0.150 nm; sp2-sp2 C-C bond has length of 0.147 nm.
During the reaction 3 double bonds are weaken to become single bond, simultaneously 1 single bond is strengthen to become double bond and two new sp3-sp3 single bond are formed.
Carbon has Van der Waals radius of 0.170 nm while the partly formed C-C bond in the transition state has distance of 0.211 nm which is already shorter than two times the Van der Waals radius.
(Fv611 (talk) How does the length of the bond being formed compare with the length of a sp3-sp3 bond? What can you infer from that?)
By analysing the change of bond length from reactant to transition state then to product and also looking at the vibration model in the transition state, the formation of two bonds in the reaction can be confirmed to be synchronous.
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole
MO diagram
This Diels-Alder reaction can give two different products via two different transition states (endo- and exo-). Two different transition state MO diagrams are shown below.
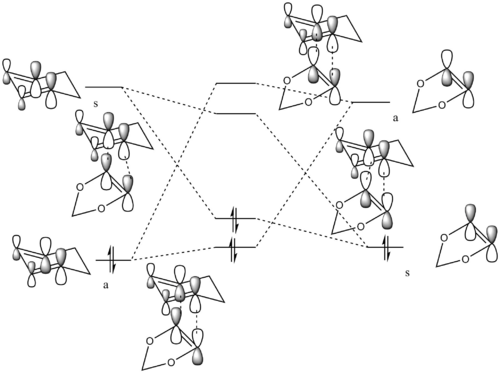 |
 |
(Fv611 (talk) Good MO's, however be careful about the placement of your reactant's fragment orbitals, as the dienophile's LUMO has the highest energy of them all.)
Energy of MOs
| Asymmetric antibonding | Symmetric antibonding | Symmetric bonding | Asymmetric bonding | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| endo- MOs | ||||||||||||
| endo- Energies | 0.01543 | -0.00462 | -0.29896 | -0.30845 | ||||||||
| exo- MOs | ||||||||||||
| exo- Energies | 0.01019 | -0.00698 | -0.18559 | -0.19800 |
| HOMO | LUMO | |||||
|---|---|---|---|---|---|---|
| Cyclohexadiene | ||||||
| Energies | -0.20598 | -0.01787 | ||||
| 1,3-Dioxole | ||||||
| Energies | -0.19617 | 0.03668 |
| HOMO | LUMO | Energy difference | |
|---|---|---|---|
| Standard | Diene | Dienophile | -0.20598-0.03668=0.24226au |
| Inverse | Dienophile | Diene | -0.01787--0.19617=0.1783au |
In this reaction, the LUMO of Diene is closer in energy with HOMO of Dienophile than opposite. Hence the reaction is an inverse demand Diels-Alder reaction.
Nf710 (talk) 22:48, 30 November 2017 (UTC) This is clever way of doing it however you must be careful when comparing NO energies of molecules on different PESs
Thermochemistry
| kj/mol | Energy of Reactants | Energy of Transition state | Reaction Barrier | Energy of Product | Reaction Energy |
|---|---|---|---|---|---|
| Endo- | -1313830.4067 | -1313622.1573 | 208.2494 | -1313849.3812 | -18.9745 |
| Exo- | -1313830.4067 | -1313614.3333 | 216.0734 | -1313845.7501 | -15.3434 |
From the data in the table above: The endo- transition state is the faster forming transition state since it has lower energy barrier, the endo- reaction also has larger reaction energy which means the endo- product is the thermodynamic product. Therefore the endo- process is both kinetically and thermodynamically favoured.
| HOMO MO diagrams with isovalue of 0.01 | |||||
|---|---|---|---|---|---|
| Endo-TS | Exo-TS | ||||
The endo- transition state has secondary orbital interaction between C=O π* orbital and C=C π orbital which provides extra stabilisation to the endo- transition state.
Nf710 (talk) 22:54, 30 November 2017 (UTC) You have calculated the energy of your reactions incorrectly I think and have therefore got the wrong answers for energy. Howver I can seee that your energy for TS and products are correct. So you have only lost 1 mark. Your discussion was very brief in this section also.
Exercise 3: Diels-Alder vs Cheletropic
IRC
| Endo- | Exo- | Cheletropic | |
|---|---|---|---|
| Animation | 
|

|
|
| IRC path | 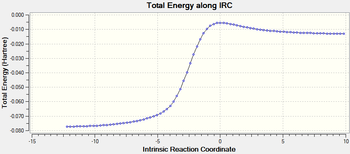
|
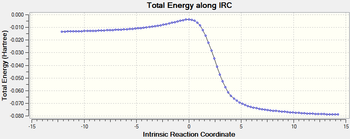
|
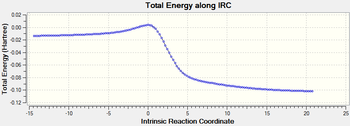
|
The six-membered ring gets aromatised during this course of the reaction.
Thermochemistry
| kj/mol | Energy of Reactants | Energy of Transition state | Reaction Barrier | Energy of Product | Reaction Energy |
|---|---|---|---|---|---|
| Endo- | 156.5979594 | 237.770549 | 81.1735896 | 56.9576013 | -99.6403581 |
| Exo- | 156.5979594 | 241.750807 | 85.1528476 | 56.3353578 | -100.2626016 |
| Cheletropic | 156.5979594 | 260.0873010 | 103.4893416 | 0 | -156.5979594 |
From the data above:
- Endo- reaction is kinetically preferred,
- Cheletropic reaction is thermodynamically preferred.
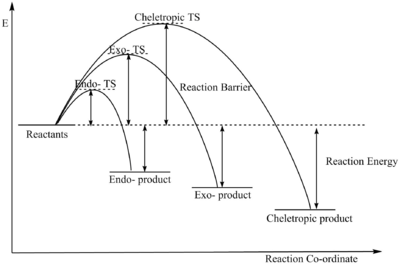
(Please avoid using curves for reaction profiles Tam10 (talk) 15:31, 27 November 2017 (UTC))
(This section is lacking discussion Tam10 (talk) 15:31, 27 November 2017 (UTC))
Conclusion
In this computational lab, Gaussian was used to determine the energy levels of MOs of the transition state of different reactions. Also by comparing the reaction barriers and reaction energies, the kinetically and thermodynamically favoured reaction pathways were determined.
File links
Exercise 1
IRC:Media:zx2015exercise1irc.log
Exercise 2
Endo- IRC:Media:zx2015exercise2endoirc.log
Exo- IRC:Media:zx2015exercise2exoirc.log
Exercise 3
Endo- Transition state:Media:zx2015exercise3endots.log
Endo- IRC:Media:zx2015exercise3endoirc.log
Exo- Transition state:Media:zx2015exercise3exots.log
Exo- IRC:Media:zx2015exercise3exoirc.log
Cheletropic Transition state:Media:zx2015exercise3cheletropicts.log
Cheletropic IRC:Media:zx2015exercise3cheletropicirc.log
