Rep:TFI15.TS
Introduction
Potential energy surface (PES) illustrates the energy of a system and can be used the study to molecular geometry and reaction dynamics of a system. It depends on the location of the atoms relative to one another and show that each geometry is associated with its own electronic potential energy. Number of dimensions in a PES depends on the number of geometric degrees of freedom of the molecules present. Geometric degrees of freedom of a molecule is determined by the number of normal vibrational modes. For example, in a non-linear molecule, there are (3N – 6) modes where N is the number of atoms.
Nf710 (talk) 23:07, 22 February 2018 (UTC) It is the other way round. The vibrations depend on the degrees of freedom. these are the eigenvectors of the hessian at a TS and these are 3N-6 linear combinations of the degrees of freedom.
Minimum of a PES represents where the reactants or products are as they have the lowest possible potential energy. The first derivatives of the minimum points give a gradient of zero and the second derivatives give positive curvatures. This is because potential energy increases as the coordinate is moved away from the minimum points. Transition state is represented as a saddle point in the PES where is located between two minimum points. It is the maximum point of the lowest energy pathway. The first derivative of the transition state gives zero gradient and the second derivative gives a negative curvature. A correct reaction coordinate can be identified if there is only one imaginary frequency as it implies that apart from the transition state, all other degrees of freedom are relaxed and in all other orthogonal directions, energy is at minimum.
Nf710 (talk) 23:10, 22 February 2018 (UTC) You get these force constants by diagonalising the hessian and getting the eigenvalues at a TS geom.
In the following report, three Diels-Alder reactions were studied. GaussView was used to construct the molecules and Gaussian was used to simulate the reactions and carried out calculation. The structures of the reactants, transition states and products were first optimised by semi-empirical PM6 method. This method involved applying empirical corrections onto experimental data and thus the calculations were fast. The structures were further optimised using B3LYP/ 6-31G(d) method to enhance the accuracy of the calculations. This method was more accurate as it was one of the Density Functional Theory methods which took in account of density fitting of the atoms. Intrinsic Reaction Coordinate (IRC) calculations were also obtained to visualise the reaction coordinate and understand the geometry of the reactants, transition states and products.
Exercise 1: Reaction of Butadiene and ethylene
Reaction Scheme:
(Fv611 (talk) Nice work in this exercise. You only missed out the discussion on the synchronicity of the bond formation in this reaction.)
Butadiene reacts with ethylene under [4 + 2] cycloaddition, which is also known as Diels-Alder (AD) reaction. Note that only cis-butadiene can undergo AD reaction with ethylene but not trans-butadiene. This is because when butadiene is in trans orientation, it does not overlap with ethylene properly and therefore will not able to form interacting orbitals. Only HOMO and LUMO of cis butadiene overlap with that of ethylene to form bonding orbitals.
1. Optimisation of Reactants, Transition State and Product
All reactants, transition states and products were optimised by semi-empirical PM6 method.
1.1. Reactants
| Butadiene | Ethylene | ||||
|---|---|---|---|---|---|
1.2. Transition State
| Transition State | ||
|---|---|---|
1.2.1. Frequency Calculations
| Transition State Vibration | Frequency Calculations |
|---|---|

|
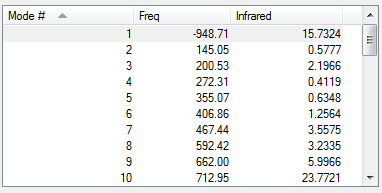
|
Only one imaginary frequency was obtained in the calculations, indicating a transition state with the correct geometry was obtained.
1.2.2. IRC of Reaction
| IRC of Reaction | IRC results |
|---|---|

|
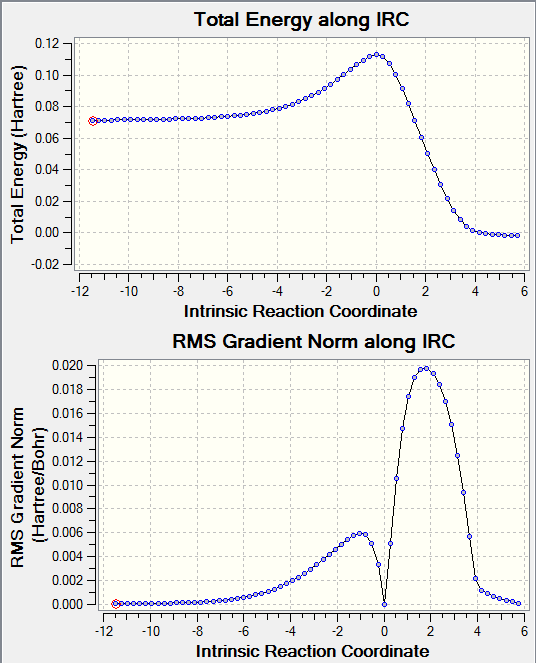
|
IRC was calculated from the transition state in both directions. Transition state is located at where the total energy is at maximum. Along the forward and backward directions total energies fall until energy levels of products or reactants are reached respectively. In the above figure, IRC of reactants to products was observed, with products lying at a lower energy level than that of reactants. RMS gradient norm along IRC shows the gradient of the total energy curve along the IRC. In the above figure, at IRC = 0, total energy was at maximum while RMS gradient norm was at 0. This indicated the successful formation of transition state.
1.3. Product
| Product | ||
|---|---|---|
Product was optimised to PM6 level. No imaginary frequency observed indicating that the structure of the of the product was optimised.
2. MO analysis
2.1 Molecular Orbitals of Reactants
| Reactants | HOMO | LUMO | ||||
|---|---|---|---|---|---|---|
| Butadiene | ||||||
| Ethylene |
2.2 Molecular Orbitals of Transition State
| HOMO -1 | HOMO | LUMO | LUMO +1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
2.3. Molecular Orbitals Diagram of Transition State
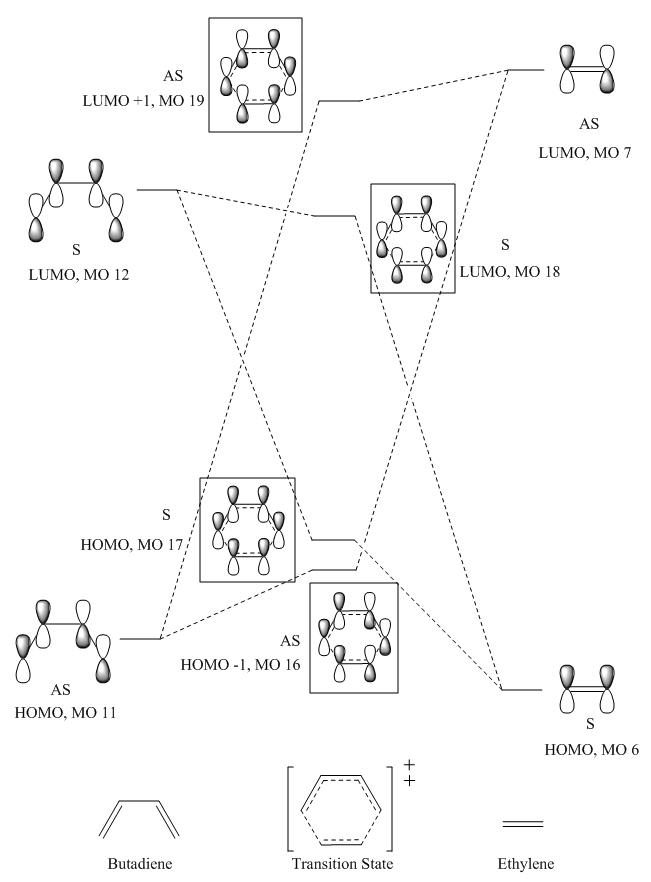
HOMO of butadiene and LUMO of ethylene interacts to give HOMO -1 and LUMO +1 orbitals of transition states, which corresponds to MO 16 and MO 19 in JSmol file. LUMObutadiene and HOMOethylene interacts to give HOMO and LUMO of transition state, which corresponds to MO 17 and MO 18 in JSmol file. The HOMO and HOMO -1 of transition state have a high energy than the HOMObutadiene and HOMOethylene. This experimental data is different from what is expected for HOMO of products, where HOMO of product are more stabilised than that of reactants. This is mainly due to orbital mixing in transition state where orbitals are closer together and delocalised. This allows electrons to move into different orbitals and the formation and new bonds. This can also be used to explain the energy level of LUMO +1 of transition state, which is of lower energy than the LUMOethylene.
It is observed that symmetrical orbitals interact with symmetrical orbitals only to give symmetrical orbitals in transition state, same observation applies to antisymmetric orbitals. It can be concluded that reaction is only allowed when the symmetries of the molecular orbitals are the same. This is due to bond formation is only allowed when the overlap integral is non-zero where components are of the same symmetry. Values of overlap integrals are illustrated as follow:
| Interactions | Overlap Integrals |
|---|---|
| symmetric - symmetric | non-zero |
| symmetric - antisymmetric | zero |
| antisymmetric - antisymmetric | non-zero |
Table 1: Values of overlap integrals regarding interactions of different symmetries.
Woodward-Hoffmann rules can be used to further explain the requirement for an Diels-Alder reaction to take place. Woodward-Hoffman rule illustrates that for a reaction to be allowed, (4n + 2)s + (4n)a must be odd. 'n' is any integer referring to the number of pi electrons. ‘s’ stands for suprafacial while ‘a’ stands for antisuprafacial. Therefore, (4n + 2)s refers to the number of terminal bonding interactions by overlap of orbitals with the same face. (4n)a refers to the number of terminal bonding interactions by overlap of orbitals with opposite faces. If (4n + 2)s + (4n)a is not an odd number, bond formation is forbidden. In the above reaction, (4n + 2)s + (4n)a = 1 + 0 = 1, thus the reaction was allowed.
3. Bond lengths
| Reactants | Transition State | Product |
|---|---|---|
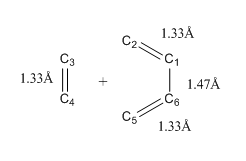
|
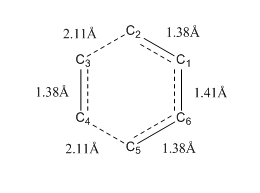
|

|
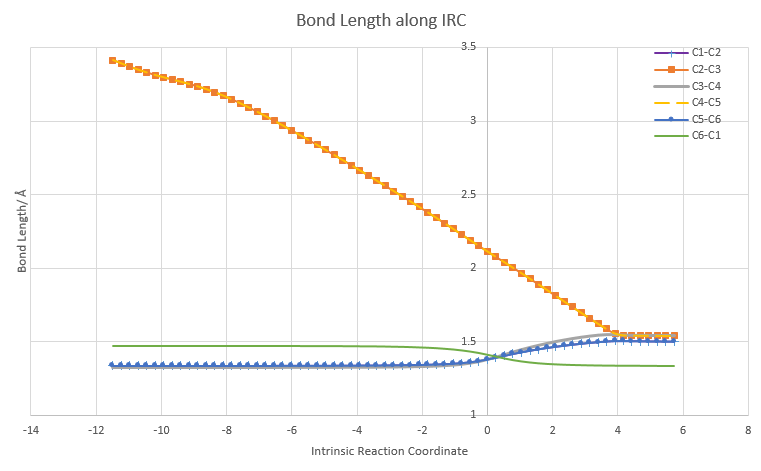
The different carbon atoms involved in the reaction were labelled as C1 to C6. Bond length along the IRC between different C atom pairs were plotted in the above figure.
| Bond Types | Typical Bond Lengths/ Å |
|---|---|
| sp2-sp2 C=C | 1.32 |
| sp3-sp3 C-C | 1.53 |
| sp2-sp2 C-C | 1.48 |
| sp3-sp2 C-C | 1.51 |
| Van der Waals distance | 1.7 |
Table 2: Typical bond lengths of different C-C bond types.[1]
The changes in bond length of two C atom pairs, C2-C3 and C4-C5 exhibited a similar trend. The bond lengths of these two pairs decreased along the IRC until a plateau was reached. C3 and C4 are the two carbon atoms of the ethene while C2 and C5 are the terminal carbon atoms on the butadiene. Bond length between C3 and C2 decreased along the IRC. This is because as the reaction proceeded, the two components became closer together thus the distance between C3 and C2 decrease. The same applied to atoms C4 and C5. At transition state, the distance between C3 and C2 is 2.11 Å, which shorter than the typical Van der Waals C-C bond length. This is because a partial bond was forming between the two atoms and thus the atoms were attracted to each other and distance was shorter. The curve reached a plateau as new carbon-carbon bond was formed between the components in the product state and the typical bond length of sp3-sp3 carbon was reached.
The changes in bond length of another two C atom pairs, C1-C2, C3-C4 and C5-C6 exhibited a similar trend. Bond length remained steady along the IRC until transition state was reached. At transition state, bond length increased until products were formed, where the plot reached a plateau. At the start of the IRC, all three pairs had a bond length of 1.33 Å, indicating that they were all sp2 hybridised with a double bond in between. At the transition state, bond length of 1.38 Å was longer than a typical sp2=sp2 C=C bond length. This is because the bond was change from a double to a single bond as the hybridisation of C atoms changed from sp2 to sp3. Thus at transition state, a partial double bond was observed. Plateau was reached at the end of the IRC as product was formed. Bond length of C3-C4 indicated that a typical sp3-sp3 C-C bond was formed. Bond length (1.49 Å) of C1-C2 and C5-C6 did not show a typical sp3-sp3 C-C bond length. This was because both C1 and C6 were sp2 hybridised. Thus the bond length exhibited characteristics of sp3-sp2 C-C bond length.
Lastly, the bond length of C1-C6 atom pair decreased during transition state. This was because the bond holding C1 and C6 was changing from a single bond to a double bond. In reactant state, the bond length was a typical sp2-sp2 C-C bond length. At transition state, bond length decreased to 1.41 Å which was in between a sp2-sp2 C-C bond and C=C bond length. This was because a partial double bond was formed as electron was delocalised from the adjacent double bond. At the plateau of the curve, typical sp2-sp2 C=C bond length was reached, indicating that a double bond was formed.
Exercise 2: Reaction of Cyclohexadiene and 1, 3-Dioxole
Reaction Scheme
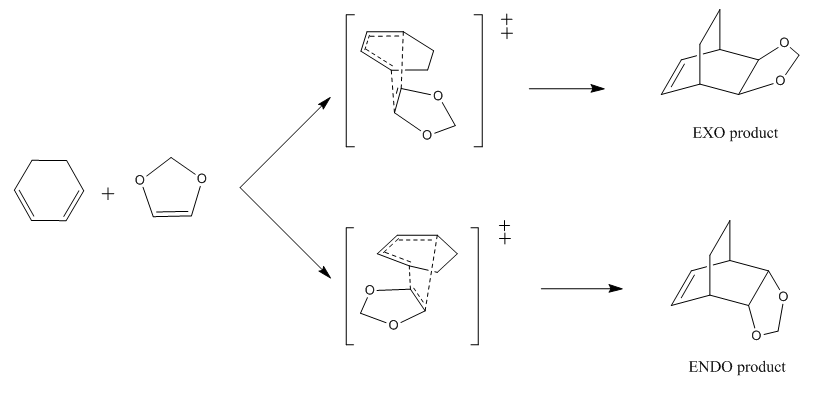
Diels-Alder reaction between cyclohexadiene and 1,3-dioxole can lead to formation of both exo and endo products. Which product forms depends on the orientation of 1,3-dioxole when approaching cycloheaxane.
1. Optimsation to B3LYP/6-31G(d) Level
In this experiment, both reactants, both exo and endo transition states and products were first optimised using PM6 method then further optimised with B3LYP/ 6-31G(d) method. Their vibration frequencies were studied to ensure that the correct structure was obtained. Only one imaginary frequency was observed in calculations for exo and endo transition states, indicated that a saddle point was reached and transition state formation was successful.
1.1 Reactants
| Results: | Cyclohexadiene | 1,3-Dioxole | ||||
|---|---|---|---|---|---|---|
| Optimised Structure | ||||||
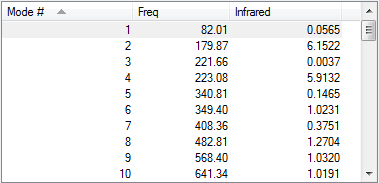
| Vibration Frequency |  |
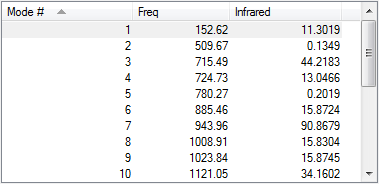 |
1.2. Transition State
| Results: | EXO | ENDO | ||||
|---|---|---|---|---|---|---|
| Optimised Structure | ||||||
| Vibration Frequency | 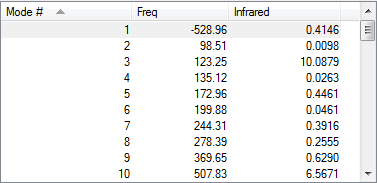 |
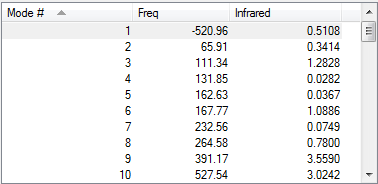 |
1.3. Products
| Results: | EXO | ENDO | ||||
|---|---|---|---|---|---|---|
| Optimised Structure | ||||||
| Vibration Frequency | 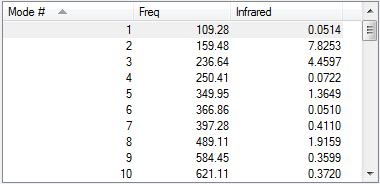 |
 |
2. MO Analysis
2.1. EXO Transition State
| HOMO -1 | HOMO | LUMO | LUMO +1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
2.2. ENDO Transition State
| HOMO -1 | HOMO | LUMO | LUMO +1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
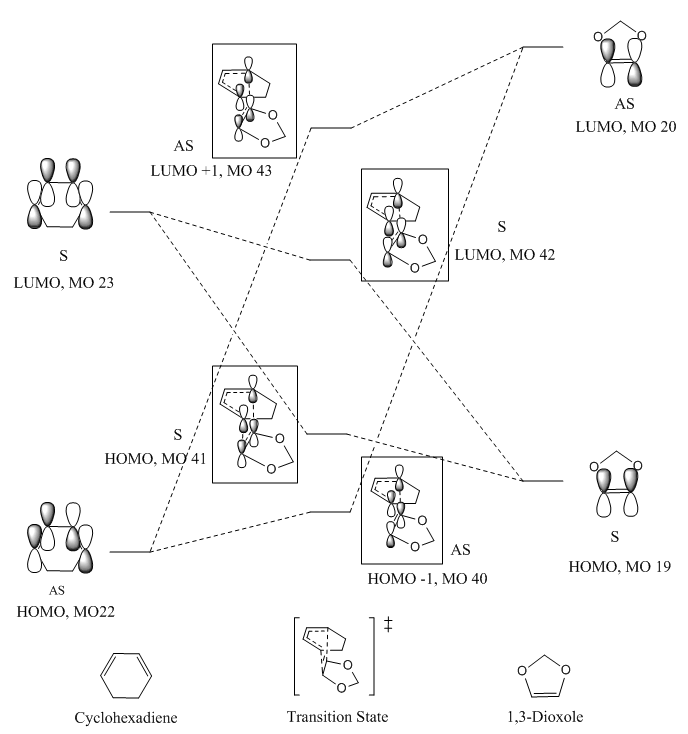
2.3. MO Diagram of EXO Transition State VS. ENDO Transition State
(Fv611 (talk) The MO diagrams are well constructed, but you could have extended the discussion and compared the relative MO energies between the endo and the exo conformations.)
| EXO | ENDO |
|---|---|
 |
 |
Molecular orbitals of both exo and endo transition states were studied. HOMO -1, HOMO, LUMO and LUMO +1 were identified as MO 40, MO 41, MO 42 and MO 43 respectively in both transition states. Through studying the experimental energies of the MO, a similar trend to DA reaction between butadiene and ethylene was found. MO 41 (ie. HOMO of transition state), which was formed by HOMO1,3-dioxole and LUMOcyclohexadiene was less stabilised than the HOMO of reactants. MO 42 (ie. LUMO of transition state) was more stabilised than the LUMO of reactants. This is mainly due to orbital mixing in Diels-Alder reactions where energy levels of molecular orbitals were brought closer together.
Nf710 (talk) 23:16, 22 February 2018 (UTC) First How do you know this. You have not given any values. Secondly its quite diffiuclt to compare MOs energies of different molecular systems as the potential terms are very different so it doesnt really make sense to do so. You should only really compare MOs if they are on the same Molecular surface.
Comparing the experimental energies of exo transition state MO and endo transition state MO, it was found that the energy difference between LUMO and HOMO of exo transition state was smaller than that of endo transition state. This will be further explained in the following energy analysis section.
There are two types of Diels-Alder reactions, namely normal reaction and inverse reaction. In a normal Diels-Alder reaction, there are electron-rich diene and electron-poor dienophile. Molecular orbitals of diene are of higher energy than that of dienophile. HOMOdiene has a similar energy as LUMOdienophile. Therefore, the two molecular orbitals interact to give bonding and antibonding orbitals in transition state. In an inverse Diels-Alder reaction, diene is less electron-rich than dienophile. Molecular orbitals of electron deficient diene are of lower energy than that of electron-rich diene. LUMOdiene is close in energy with HOMOdienophile and thus they interact to form bonding and antibonding orbitals in transition state.
Referring to the MO diagram of exo and endo transition states, it can be concluded that both reactions are inverse Diels-Alder reactions. 1,3-dioxole was acting as an electro-rich dienophile. This was because the two oxygen atoms adjacent to the C=C bond were strong electron donating groups which raised the energy levels of 1,3-dioxole. Therefore, 1,3-dioxole was of higher energy than cyclohexadiene. HOMO1,3-dioxole and LUMOcyclohexadiene are closer in energy than HOMOcyclohexadiene and LUMO1,3-dioxole. Thus they are the frontier molecular orbitals which interact strongly.
It is best to compare the MOs energies of the reactants by doing a single point energy calculation with both reactanats on the same surface (the first geom on the irc)
2.4. Sum of Electrical and Thermal Free Energies
Reactants, transition states and products were optimised to B3LYP/6-31G(d) level and the Gibbs free energies were recorded. Activation energy was the energy difference between the sum of reactants and transition states. Reaction energy was the energy difference between sum of reactants and products.
| Structures | ENDO-DA Gibbs Free Energy/ KJ mol-1 | EXO-DA Gibbs Free Energy/ KJ mol-1 |
|---|---|---|
| 1,3-Dioxolene | ||
| Cyclohexadiene | ||
| Sum of Reactants | ||
| Transition State | -1313622.15 | -1313614.33 |
| Products | -1313849.37 | -1313845.78 |
| Activation Energy | 159.82 | 167.64 |
| Reaction Energy | -67.40 | -63.81 |
Table 7: Gibbs free energies of molecules in endo and exo Diels-Alder reactions.
It was found that activation energy of endo Diels-Alder reaction was lower than that of exo reaction. The energy barrier that was needed to be overcome in order to obtain products was lower for endo reaction. Thus, endo product was more kinetically favourable. Moreover, the reaction energy of endo reaction was more negative than that of exo reaction. This implied that endo product formed was more stable than exo product. Thus endo Diels-Alder reaction was also more thermodynamically favourable.
2.5. Secondary Orbital Interaction
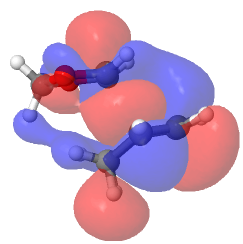
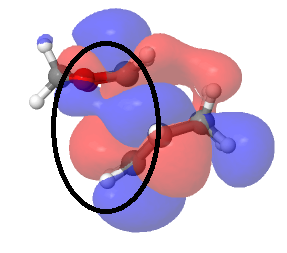
Endo Diels-Alder reaction was favoured both kinetically and thermodynamically. This could be explained through secondary orbital interaction and structure of the products. By observing the HOMO of transition state with isovalue of 0.01, it was found that secondary orbital interaction was only present in endo transition state but absent in exo transition state. Secondary orbital interaction was present between the back lobes of cyclohexadiene and the lone pair on oxygen atoms of 1,3-dioxole. This was only allowed in endo transition state as the C=O lobes were underneath the C=C lobes while in exo transition state, C=O pointed away from the cyclohexadiene. On top of primary orbital interaction which led to formation of sigma bonds, secondary orbital interaction stabilised the transition state. Therefore, endo approach was kinetically favoured as the transition state energy was lower.
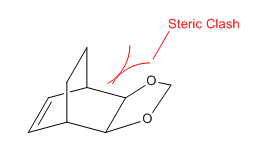
In addition, by looking at the structure of the exo product, it could be concluded that steric clash was present in exo product but not in endo product. There was a steric clash in exo product between the carbon bridging group and the dioxole groups as they pointed towards the same direction. Therefore, exo product had a higher energy than that of endo product and the reaction energy was less negative. This implied that endo Diels-Alder approach was more thermodynamically favourable than the exo approach.
Nf710 (talk) 23:21, 22 February 2018 (UTC) This section of ok, you could have quantitatively probe if it was inverse or normal. But your energies are correct and you have come to the correct conclusions. you could have going into more of the theory about the thermodynamics.
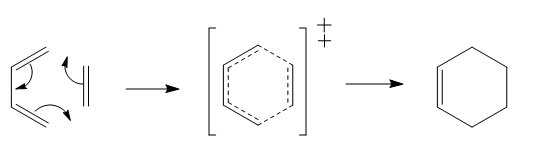
Exercise 3: Cycloaddition of o-xylylene and SO2
Reaction Scheme
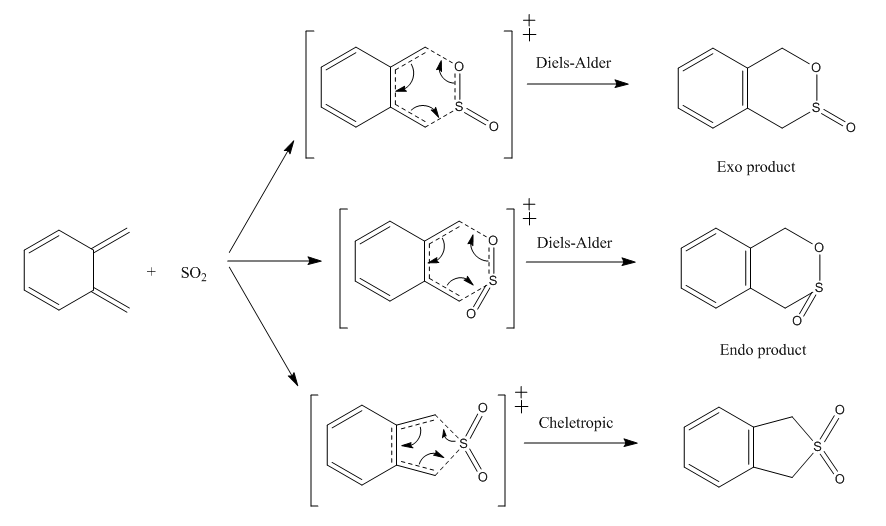
SO2 and o-xylylene can react via both Diels-Alder and Cheletropic reaction pathways. There are two butadiene group in o-xylylene which can react with SO2. Reaction between external butadiene and SO2 was first studied.
1. Optimsation to PM6 Level and IRC Calculations
| Structures: | Optimised Transition State | IRC | Total Energy along IRC | ||
|---|---|---|---|---|---|
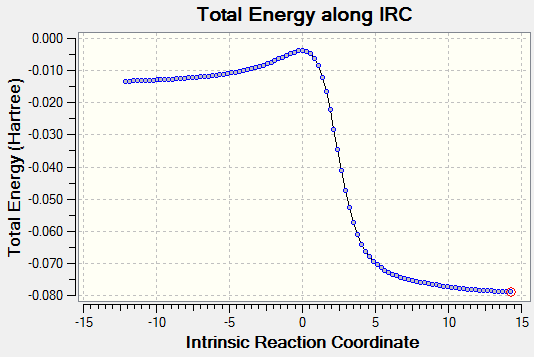
| EXO Diels-Alder | 
|
 | |||
| ENDO Diels-Alder | 
|
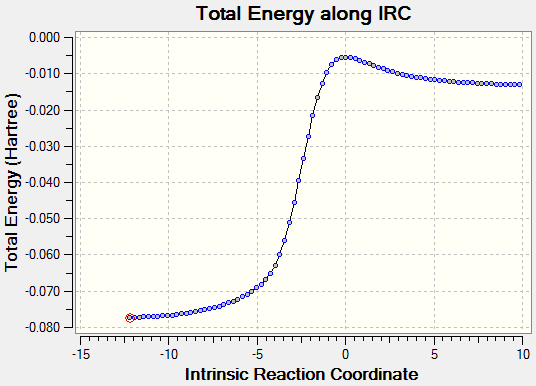 | |||
| Cheletropic | 
|
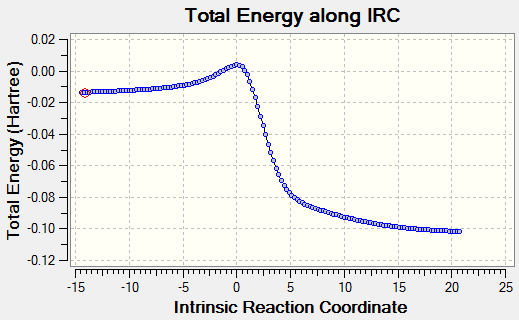 |
Transition states for the Cheletropic, endo and exo Diels-Alder reactions were optimised at the PM6 level. IRC calculations and total energy along IRC for each path were obtained. By visualising the IRC, it was found that despite the internal butadiene was not involved in the reaction with SO2, a partial double bond was observed throughout the 6-membered ring of o-xylylene. This shows that electrons were delocalised throughout the entire o-xylylene instead of only in between external butadiene and SO2. The planar 6-membered ring gained aromaticity and led to the formation of a benzene ring. Benzene ring was more stable than the o-xylylene due to delocalisation of electrons in the ring.
2. Energy Analysis
2.1. Sum of Electrical and Thermal Free Energies
| Structures | ENDO-DA Gibbs Free Energy/ KJ mol-1 | EXO-DA Gibbs Free Energy/ KJ mol-1 | Cheletropic Gibbs Free Energy/ KJ mol-1 |
|---|---|---|---|
| O-Xylylene (external) | |||
| SO2 | |||
| Sum of Reactants | |||
| Transition State | 237.77 | 241.75 | 260.09 |
| Products | 56.98 | 56.32 | 0.01313 |
| Activation Energy | 82.04 | 86.02 | 104.36 |
| Reaction Energy | -98.74 | -99.40 | -155.71 |
Table 8: Gibbs free energies of molecules in Cheletropic, endo and exo Diels=Alder reactions.
2.2 Energy Profile of EXO-Diels Alder, ENDO-Diels Alder and Cheletropic Reactions
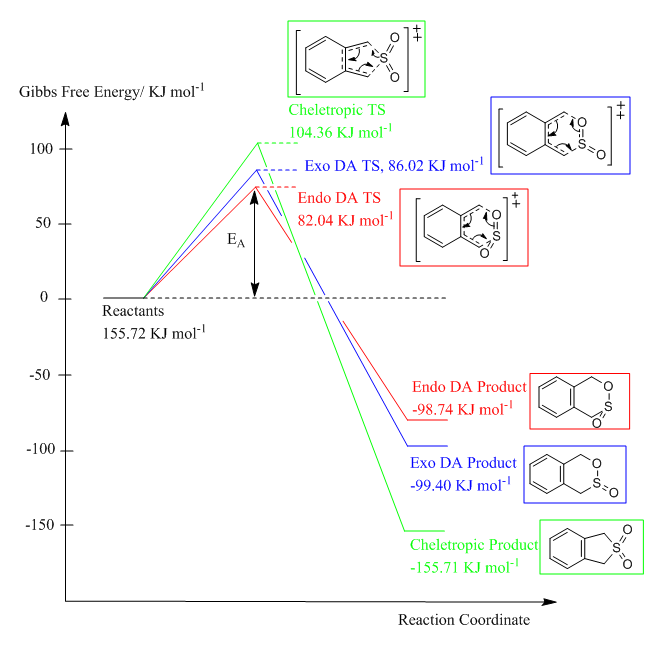
Energies of the reactants, transition states and products from the Cheletropic, endo and exo Diels-Alder reactions were obtained. Energy profile showing the relative heights of the energy levels of the reactants, transition states and products were plotted. It was found that the activation energy for Cheletropic reaction was higher than the Diels-Alder reaction. Therefore, Diels-Alder reactions were more kinetically favourable. Among the two Diels-Alder reaction, endo pathway was more kinetically favourable as it had a lower activation energy than the exo pathway. This is because secondary orbital interaction is present in the endo transition state which stabilises the transition state and lowers its energy.
On the other hand, reaction energy of Cheletropic reaction was the most negative, indicating that Cheletropic pathway was more thermodynamically favourable than the Diels-Alder reaction. This was due to the strong S=O bond present in SO2 which tend to maintain the strong bond throughout the reaction. Product with two S=O structure maintained is more stable and thus lies at a lower energy level. Endo Diels-Alder pathway was more thermodynamically unfavourable than Exo pathway as it had a less negative reaction energy.This is because there is a larger steric clash in the endo product than the exo product which makes the endo product in a higher energy level than that of exo product.
2.3. Reaction betweem Internal Butadiene of o-xylylene and SO2
| Reaction Scheme |
|---|
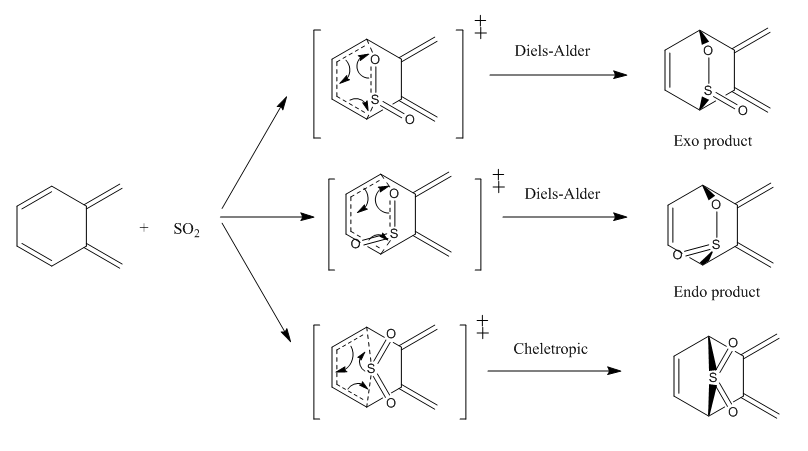 |
Reaction between internal butadiene of o-xylylene and SO2 was also investigated. IRC of the reactions were visualised and energies of the reactants, transition states and products for each Cheletropic, endo and exo Diels-Alder reactions were obtained.
| IRC of ENDO-DA Reaction | IRC of EXO-DA Reaction | IRC of Cheletropic Reaction |
|---|---|---|

|

|

|
| Structures | ENDO-DA Gibbs Free Energy/ KJ mol-1 | EXO-DA Gibbs Free Energy/ KJ mol-1 | Cheletropic Gibbs Free Energy/ KJ mol-1 |
|---|---|---|---|
| O-Xylylene (internal) | |||
| SO2 | |||
| Sum of Reactants | |||
| Transition State | 267.98 | 275.82 | 296.68 |
| Products | 172.26 | 176.70 | 203.28 |
| Activation Energy | 112.26 | 120.10 | 140.96 |
| Reaction Energy | 16.54 | 21.00 | 47.56 |
Table 9: Gibbs free energies of molecules in Cheletropic, endo and exo reactions between internal butadiene of o-xylylene and SO2.
It was found that reaction with internal butadiene was both kinetically and thermodynamically unfavourable. The activation energies were much higher than the reactions involving external butadiene and were therefore kinetically unfavourable. This is because there is no gain in aromaticity and formation of benzene in the 6 membered ring. Therefore, there is a smaller driving force for the reaction to take place as compared to that of reaction involving external butadiene in o-xylylene. Reaction energies were positive indicating that the reactions were endothermic and the products formed were of high energies. This was mainly due to a severe steric clash in the reaction of SO2 with internal butadiene of 6 membered ring. This can be seen through the IRC where the oxygen on SO2 clashed with the external butadiene of o-xylylene. As a result, reactions with internal butadiene were also thermodynamically unfavourable.
Conclusion
Simulation done by semi-empirical PM6 and BeLYP/ 6-31G(d) methods gave accurate results on the structures, geometries and energies of the molecules involved in a reaction. IRC was also an useful tool in visualising the reaction profile.
It was found that [4 + 2] cycloaddition of cis-butadiene and ethene follows a normal Diels-Alder reaction scheme as cis-butadiene is an electron-rich diene while ethene is an electron-deficient dienophile.
It was also found that cyclohexadiene and 1,3-dioxole undergo inverse Diels-Alder reaction. This is because 1,3-dioxole is an electron-rich dienophile which has a higher energy than that of cyclohexadiene.
Finally, it was found that while SO2 can undergo both Diels-Alder and Cheletropic reactions with o-xylylene, endo Diels-Alder pathway are both kinetically and thermodynamically favoured. In addition, reaction with external butadiene in o-xylylene is preferred over internal butadiene.
Cycloadditions are important and useful reactions to form new C-C bond and link molecules together. Gaussian is an useful tool in predicting the reaction profile.
References
[1] H.J. Bernstein, Bond Distances In Hydrocarbons, The Faraday Society and Contributors, 1961