Rep:Ss2310:Module 3
Module 3
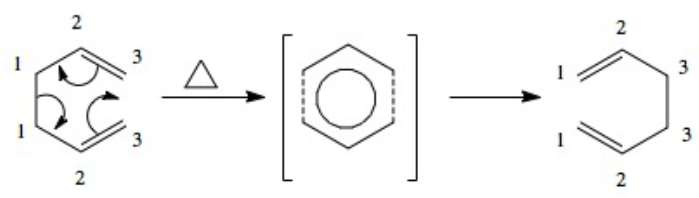
Cope Rearrangement: [3,3] sigmatropic shift
The above mechanism shows that there is a flow of 6 electrons during the rearrangement process. According to the Huckel rule (4n+2) this means that the intermediate transition state possesses aromatic character and is therefore more stabilised energetically due to favourable delocalisation. Interestingly, the intermediate also has higher symmetry than either the reactant or product.
Optimising 'anti' 1,5-hexadiene
The first step taken was to draw 1,5-hexadiene in Guassview and ensure that central 4 carbons had anti-periplanar conformation. Structure was 'cleaned' and then optimised. The calculation set-up was as follows: 1. Job Type: Optimisation 2. Method: Hartree-Fock, 3-21G basis set 3. Link 0: mem%500MB 4. Once the job had completed the chk file was opened.
| Conformer | Energy(a.u.) | Point group (Symmetrised) | Dipole moment (Debeye) | Structure |
|---|---|---|---|---|
| anti 2 | -231.69253529 | Ci | 0.0002 |  |
| gauche 2 | -231.69166696 | C2 | 0.3799 |  |
Job convergence information:
(i) Anti 2
Item Value Threshold Converged?
Maximum Force 0.000034 0.000450 YES
RMS Force 0.000007 0.000300 YES
Maximum Displacement 0.001226 0.001800 YES
RMS Displacement 0.000346 0.001200 YES
Predicted change in Energy=-1.334886D-08
Optimization completed.
-- Stationary point found.
(ii) Gauche 2
Item Value Threshold Converged?
Maximum Force 0.000118 0.000450 YES
RMS Force 0.000024 0.000300 YES
Maximum Displacement 0.001511 0.001800 YES
RMS Displacement 0.000383 0.001200 YES
Predicted change in Energy=-8.082607D-08
Optimization completed.
-- Stationary point found.
Lowest Energy Conformation
| Conformer | Energy(a.u.) | Point group (Symmetrised) | Dipole moment (Debeye) | Structure |
|---|---|---|---|---|
| anti 1 | -231.69260235 | C2 | 0.2021 |  |
| gauche 1 | -231.68771617 | C2 | 0.4557 |  |
Job convergence information:
(i) anti 1
Item Value Threshold Converged?
Maximum Force 0.000056 0.000450 YES
RMS Force 0.000010 0.000300 YES
Maximum Displacement 0.001565 0.001800 YES
RMS Displacement 0.000459 0.001200 YES
Predicted change in Energy=-2.090792D-08
Optimization completed.
-- Stationary point found.
(ii) gauche 1
Item Value Threshold Converged?
Maximum Force 0.000008 0.000450 YES
RMS Force 0.000002 0.000300 YES
Maximum Displacement 0.000242 0.001800 YES
RMS Displacement 0.000084 0.001200 YES
Predicted change in Energy=-1.777507D-09
Optimization completed.
-- Stationary point found.
Structure Comparison and Identification When comparing the experimentally calculated values to those stated in Appendix 1, there is close agreement in the values up to approximately 5d.p. The second lowest energy conformers (anti and gauche 2) have total Hartree/atomic unit energies of -231.69253529 and -231.69166696. The stated values for anti 2 and gauche 2 are -231.69254 and -231.69167. There is also a change in energy of -1.334886D-08 and -8.082607D-08 when optimising from the starting structure. The large difference between the anti and gauche conformers can be rationalised when taking into account the unfavourable repulsion between the C2H5 groups when lying at 60 degrees (gauche) to each other. This interaction is repulsive and creates a higher energy barrier compared to the eclipsed anti-periplanar conformer.
Ci anti2 1,5-hexadiene optimisation using B3LYP/6-31G* Basis Set
Reoptimised 3-21G structure using higher B3LYP/6-31G* basis set. Calculation was carried out as follows: 1. Job: Optimisation 2. Method: Selected DFT and B3LYP 3. Link 0: Changed chk file name to that of DFT opt file.
| Basis Set | Energy(a.u.) | Point group | Dipole moment (Debeye) | Structure |
|---|---|---|---|---|
| 3-21G | -231.69253529 | Ci | 0.0002 |  |
| 6-31G* | -234.55970424 | Ci | 0.0000 |  |
The overall geometry does not change significantly but there is a slight rotation of the central two carbons (C3 and C4). Using the higher 6-31G* basis set has resulted in a lower energy conformer -234.55970424 when compared to the simpler 3-21G basis set which gives an energy of -231.69253529. Interestingly, after optimising at this higher level, Gaussian now states that there is no longer a dipole moment and hence no accumulation of positive/negative charge at a one particular point on the molecule.
Anti 2 HF/3-21G Thermochemistry information:
Sum of electronic and zero-point Energies= -231.539539 Sum of electronic and thermal Energies= -231.532566 Sum of electronic and thermal Enthalpies= -231.531621 Sum of electronic and thermal Free Energies= -231.570913
When comparing the experimental results to those states, the energy values for 'Sum of electronic and zero-point Energies' are both -231.539539 hartree. There is also an agreement up to 6d.p in the overall Electronic Energy values at -231.692535 and -231.69253529 respectively for stated and experimental values. There is also agreement between the experimentally calculated values of -231.532566 hartree for 'Sum of electronic and thermal Energies' and the literature stated values of -231.532566 hartree.
Low frequencies --- -2.6036 -1.8534 -1.5120 0.0004 0.0008 0.0008 Low frequencies --- 71.3545 85.7097 116.2084
First Frequency Value: 71.35cm-1
Job convergence information: (i) 3-21G
Item Value Threshold Converged?
Maximum Force 0.000034 0.000450 YES
RMS Force 0.000007 0.000300 YES
Maximum Displacement 0.001226 0.001800 YES
RMS Displacement 0.000346 0.001200 YES
Predicted change in Energy=-1.334886D-08
Optimization completed.
-- Stationary point found.
(i) 6-31G*
Item Value Threshold Converged?
Maximum Force 0.000079 0.000450 YES
RMS Force 0.000019 0.000300 YES
Maximum Displacement 0.000471 0.001800 YES
RMS Displacement 0.000196 0.001200 YES
Predicted change in Energy=-8.950676D-08
Optimization completed.
-- Stationary point found
6-31G* anti2 1,5-hexadiene Frequency Analysis
After re-optimising the HF/3-21G anti 2 structure to 6-31G*, a frequency analysis was also carried out and results compared to the given values. The calculation was carried out as follows: 1. Gaussian calculation set-up, Job Type: Frequency 2. Change chk. file name under Link 0 tab so that it is not overwritten. 3. Ensure that 'scrf=(solvent=water,check)' is not part of the method and then submit job. 4. Open log file once job was run and visualise vibrations.
Link to anti2 1,5-hexadiene 6-31G* frequency file: [7]
First Frequency value: 93.84cm<sup>-1</sup>
The absence of a negative frequency values shows that imaginary frequencies are absent in this structure.
Low frequencies --- -0.0008 -0.0008 -0.0006 21.2228 29.2342 63.2481 Low frequencies --- 93.8433 101.9293 138.2437
Sum of electronic and zero-point Energies= -234.469219 Sum of electronic and thermal Energies= -234.461966 Sum of electronic and thermal Enthalpies= -234.461021 Sum of electronic and thermal Free Energies= -234.500370
The 'Electronic energy' experimental value is -234.55970424 hartree which is lower than the -234.611710 hartree value states for anti 2 when running a frequency analysis at the higher basis set B3LYP/6-31G* theoretical set-up. The values for'Sum of electronic and zero-point Energies' are in agreement up to 4d.p. with the experimental calculation of -234.469219 compared to -234.469203, which suggests that the experimentally determined structure had a higher than expected potential energy at 0K when including the zero-point vibrational energy. Similarly, there is also agreement of up to 4d.p in hartree when comparing 'Sum of electronic and thermal Energies' with previously stated values of -234.461856 which is again slightly lower than the experimentally determined value of -234.461966. This may suggest that the experimental conformer experienced greater contributions in terms of translational, rotational, and vibrational energy modes due to not having undergone an ideal optimisation.
Optimising 'Chair' and 'Boat' Transition Structures
An allyl fragment (CH2CHCH2) was drawn and then optimised using the HF/3-21G level of theory. A new GaussView window was then opened and the allyl structure copied into it twice. The two fragments were then manipulated in such a way that they were orientated in a chair (C2h) transition state. Once this was done, the guess molecule was copied and pasted into a New MolGroup window and a TS optimisation set up using the HF/3-21G method. This time the calculation was carried out as follows:
1. Job Type: Opt+Freq 2. Optimisation: TS (Berny), Force constants: Once 3. Additional keywords: Opt=NoEigen. 4. Submitted the job and once finished, visualised the vibrations
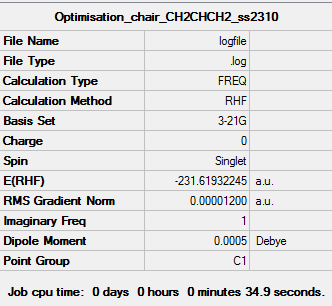
Optimised Chair (C2h) conformation
Link to optimised 'Chair' transition state file [8]
Job convergence information:
Item Value Threshold Converged?
Maximum Force 0.000010 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000625 0.001800 YES
RMS Displacement 0.000176 0.001200 YES
Predicted change in Energy=-3.648416D-08
Optimization completed.
-- Stationary point found.
Results Summary:
Imaginary Frequency Magnitude: -817.93cm-1
The chair transition structure was optimised again using the 'frozen coordinate' method. Like before, the guess structure was copied and pasted into a New GaussView window. This time the Redundant Coord Editor option was selected from the Edit menu. The 'Add Unidentified' tab was used to selected the terminal carbons that were involved in bond forming/breaking during the rearrangement process. Once the two pairs have been chosen, 'Bond' was specified below instead of 'Unidentified' and the option to Free Coordinates instead of adding them was chosen. At this point, the bonds were NOT set at the optimal distance of 2.2 Angstroms but instead this was achieved by using the 'bond' tool in the main GaussView window.This time the calculation was run slightly differently:
1. Job Type: Optimisation, to a minimum not a TS (Berny). 2. Check that the keyword 'Opt=ModRedundant' is part of the input line and submit the job.
The chk. file was opened once the calculation had run and the bond distances themselves (previously set to 2.2 Angstroms' were now optimised. The same set-up was used as before but this time when selecting terminal carbon atoms, the option 'derivative' was chosen instead of 'add'. A TS (Berny) optimisation was run but the force constants are set to 'Never'.
Link to 'frozen coordinate' chair opt file: [9]
Job convergence information:
Item Value Threshold Converged?
Maximum Force 0.000014 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000206 0.001800 YES
RMS Displacement 0.000063 0.001200 YES
Predicted change in Energy=-1.637393D-08
Optimization completed.
-- Stationary point found.
Results Summary:
Thermochemistry information:
Sum of electronic and zero-point Energies= -231.466701 Sum of electronic and thermal Energies= -231.461341 Sum of electronic and thermal Enthalpies= -231.460397 Sum of electronic and thermal Free Energies= -231.495207
The 3-21G Chair experimental optimisation has an electronic energy of -231.61932245 which agrees to 6d.p with the literature value of -231.619322. Interestingly, the frozen co-ordinate optimisation approach results in a lower energy value of -231.61932239, differing only in the last two decimal places. In terms of 'Sum of electronic and zero-point Energies' there is close agreement between the literature and experimental values up to 5d.p with results of -231.466705 hartree and -231.466701 hartree respectively. Similarly,for 'Sum of electronic and thermal Energies', the experimental value was -231.461341 hartree compared to a theoretical value of -231.461346 in Appendix 2.
Optimised Boat (C2v) conformation
To optimise the boat conformation, the QST2 method was used. This allowed us to locate the reactants and products and find the transition state between the two by interpolation. Firstly, the chk. file belonging the anti 2 Ci optimised molecule was opened and copied into a new MolGroup. From the File menu, selected 'New' and 'Add to MolGroup'. This brought up a new window, where the molecule was again copied and pasted. Clicking the arrow in the window bar, allowed both molecules to be seen. The individual atoms on each molecule were renumbered by selecting 'Edit' and 'Atom List'. The two structures were then orientated as shown below.
The calculation was set-up as follows:
1. Job Type: Opt+Freq 2. Optimisation: TS (QST2).
Failed opt job:
Link to optimised boat transition structure: [10]
However, the job fails because rotation about the central atoms of the allyl fragment was not considered and hence simply translated it, instead of optimisation. This time, the central C-C-C-C dihedral angle must be adjusted to take this problem into account. Therefore, the dihedral angle for the reactant molecule of atoms C2-C3-C4-C5 was set to 0.0. Then the C2-C3-C4 and C3-C4-C5 angles were selected and reduced to 100 degrees. Once the optimisation set-up was run again, the following structure was formed.
Successful opt job:
Link to optimised boat transition structure: [11]
Results Summary:
Imaginary Frequency Magnitude:-840.00cm-1
Thermochemistry information:
Sum of electronic and zero-point Energies= -231.450929 Sum of electronic and thermal Energies= -231.445301 Sum of electronic and thermal Enthalpies= -231.444357 Sum of electronic and thermal Free Energies= -231.479774
The 3-21G optimised boat conformer has an experimental electronic energy of -231.66280246 which is much higher than the literature value of -231.602802 which suggests that the optimisation using the QST2 method did not work fully. The experimental values for the optimised boat transition state correspond to the literature values stated in Appendix 2. Both 'Sum of electronic and zero-point Energies' and 'Sum of electronic and thermal Energies' have values of -231.450929 hartree and -231.445301 hartree. There is a small discrepancy in the 'Sum of electronic and thermal Energies' information of 0.000001 hartree.
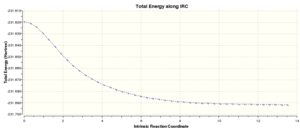
Chair: Intrinsic reaction coordinate
In order to locate the local minimum on a potential energy surface, an Intrinsic Reaction Coordinate calculation was carried out. This is achieved by geometrically moving towards a point where the slope of the energy surface is steepest until a minimum is located. The calculation was set-up as follows: (i) Job type: IRC, forward only, compute 50 steps (ii) Method: Hartree-fock, 3-21G (iii) Link O: Specify - chair_optimised.chk file (iv) Solvation: none (v) General: Write connectivity ticked, Guess: Read checkpoint file
Once the job had run, the chk. file was opened. The IRC gradient had not yet reached zero which meant that a minimum had not been located. One way to solve this was to select the last point on the graph, copy the corresponding structure into a new GaussView window and run another minimisation. Whilst this approach is fastest, there is a danger of locating the wrong minimum
File:LAPTOPRUN CHAIR IRC - COPY.LOG
Results Summary:
Job convergence information:
Item Value Threshold Converged?
Maximum Force 0.000010 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000300 0.001800 YES
RMS Displacement 0.000091 0.001200 YES
Predicted change in Energy=-2.407459D-09
Optimization completed.
-- Stationary point found
Activation Energies
The activation energies for both the chair and boat transition states were then calculated using a frequency analysis at B3LYP/6-31G* level of theory. Both structures were first optimised using the higher basis set and then the Job Type was changed to Freq.
Re-opt info for chair at 6-31G(d)
Link to optimisation file: [12]
Job convergence information:
Item Value Threshold Converged?
Maximum Force 0.000011 0.000450 YES
RMS Force 0.000002 0.000300 YES
Maximum Displacement 0.000177 0.001800 YES
RMS Displacement 0.000031 0.001200 YES
Predicted change in Energy=-3.140358D-09
Optimization completed.
-- Stationary point found.
Results Summary:
Low frequencies --- -565.5826 0.0009 0.0009 0.0010 21.9110 27.1727 Low frequencies --- 39.7535 194.4806 267.9169
Thermochemistry data:
Sum of electronic and zero-point Energies= -234.414929 Sum of electronic and thermal Energies= -234.409008 Sum of electronic and thermal Enthalpies= -234.408064 Sum of electronic and thermal Free Energies= -234.443815
The experimental thermochemistry values are slightly for 6-31G* optimised chair conformer closely agree than those given in Appendix 2. The experimental electronic energy is -234.55698303 which agrees to 6d.p with the stated figure of -234.556983. For example, the 'Sum of electronic and zero-point Energies' for experimental and theoretical are -234.414929 hartree and -234.414919 hartree. Similarly, the 'Sum of electronic and thermal Energies' show a discrepancy between stated and calculated values with -234.408998 hartree and -234.409008 hartree respectively.
Re-opt info for boat at 6-31G(d)
Link to optimisation file: [13]
Job convergence information:
Item Value Threshold Converged?
Maximum Force 0.000028 0.000450 YES
RMS Force 0.000008 0.000300 YES
Maximum Displacement 0.000636 0.001800 YES
RMS Displacement 0.000093 0.001200 YES
Predicted change in Energy=-7.644997D-08
Optimization completed.
-- Stationary point found.
Results Summary:
Low frequencies --- -530.9265 -8.8127 -0.0003 0.0006 0.0007 15.4957 Low frequencies --- 17.3620 135.3052 261.7740
Thermochemistry data:
Sum of electronic and zero-point Energies= -234.402335 Sum of electronic and thermal Energies= -234.396001 Sum of electronic and thermal Enthalpies= -234.395057 Sum of electronic and thermal Free Energies= -234.431745
The boat transition state energy values show a similar discrepancy as the chair conformer. The experimental 6-31G* optimisation method gave an experimental electronic energy of -234.54309280 compared to the value in Appendix 2 of -234.543093. However, for the corresponding values the experimental values are lower than that of the literature values in Appendix 2. For example, the 'Sum of electronic and zero-point Energies' is -234.402335 hartree compared to -234.402340 hartree. Also, the 'Sum of electronic and thermal Energies' have a difference also of -234.396001 hartree compared to a theoretical energy value of -234.396006.
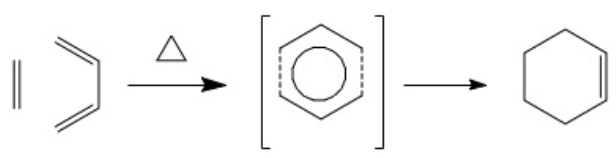
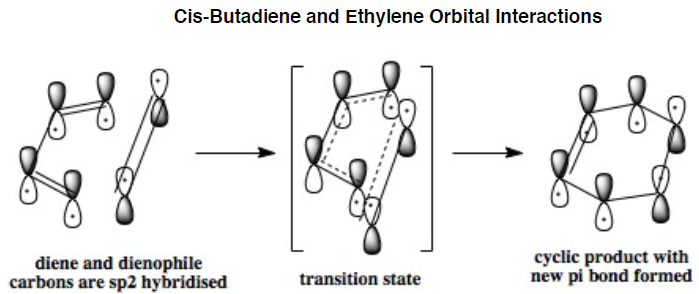
Diels-Alder Rearrangement: [4+2] cycloaddition
The above mechaniss demonstrates the movement of 6 electrons when breaking pi bonds and forming two new sigma bonds and a pi bond. Specifically, 4 electrons come from the conjugated diene (e.g. cis-butadiene or cyclohexa-1,3-diene ) and 2 electrons from a dienophile (e.g. ethylene or maleic anhydride) when taking part in the thermally allowed [4+2] cycloaddition.
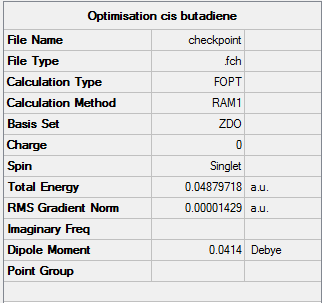
Building cis-Butadiene
A molecule of cis-butadiene was built in Gaussian and optimised using the semi-empirical, AM1 method.In order to visualise the MOs a frequency calculation was carried out. Once the job had completed, the chk. file was opened, 'Edit' tab selected and 'MO' option chosen. 1,3-butadiene can take two planar structures: 'cis' and 'trans', with the trans structure being less sterically hindered.
Link to optimised cis-butadiene file: [14]
Job convergence info:
Item Value Threshold Converged?
Maximum Force 0.000025 0.000450 YES
RMS Force 0.000011 0.000300 YES
Maximum Displacement 0.000318 0.001800 YES
RMS Displacement 0.000128 0.001200 YES
Predicted change in Energy=-8.152974D-09
Optimization completed.
-- Stationary point found.
Results Summary:
Low Frequencies of Diels-Alder reaction:
Low frequencies --- -480.6077 -12.2005 -5.2043 0.0012 0.0012 0.0012 Low frequencies --- 21.7095 136.3436 220.7358
Imaginary Frequency values: 1 -480.61 8.4073
Results Summary for Frequency:
cis-Butadiene and Ethylene Transition state
The next step after visualising the MOs of cis-butadiene was to carry out an optimisation of the transition state pathway. An approximate transition structure that had an envelope-like form was drawn in GaussView. This was carried out by first using a bicyclo structure and then removing the extra fragment CH2CH2 below. The remaining ethylene fragment was positioned at a distance of 2.2 Angstroms from the cis-butadiene and a dotted bond drawn between the terminal carbons. An optimisation and then frequency calculation using semi-empirical AM1 theory was carried out on the structure. The presence of a Diels-Alder transition state could be confirmed due to the imaginary frequency value of -480cm-1 obtained in the 'Vibrations' list. This result is indicative of a transition state as a 'minimum' value would be positive (i.e. square root of a positive energy slope) whereas this imaginary (i.e. square root of a negative energy slope)
Link to optimised TS: [ http://hdl.handle.net/10042/21315]
Job convergence info:
Item Value Threshold Converged?
Maximum Force 0.000026 0.000450 YES
RMS Force 0.000009 0.000300 YES
Maximum Displacement 0.000563 0.001800 YES
RMS Displacement 0.000227 0.001200 YES
Predicted change in Energy=-2.092648D-08
Optimization completed.
-- Stationary point found
Results Summary:
We can confirm that a transition state has been located due to the presence of an imaginary frequency value: -480cm-1
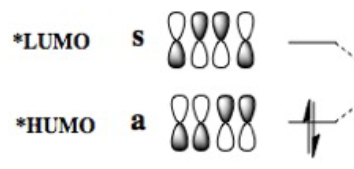
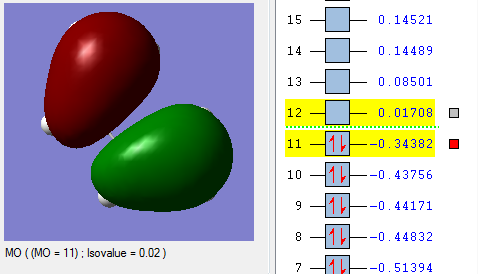
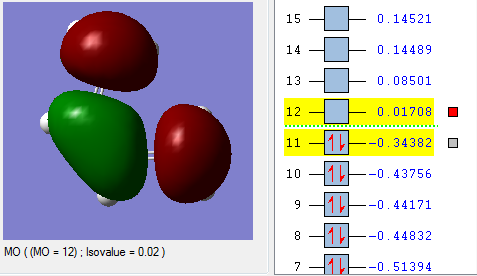
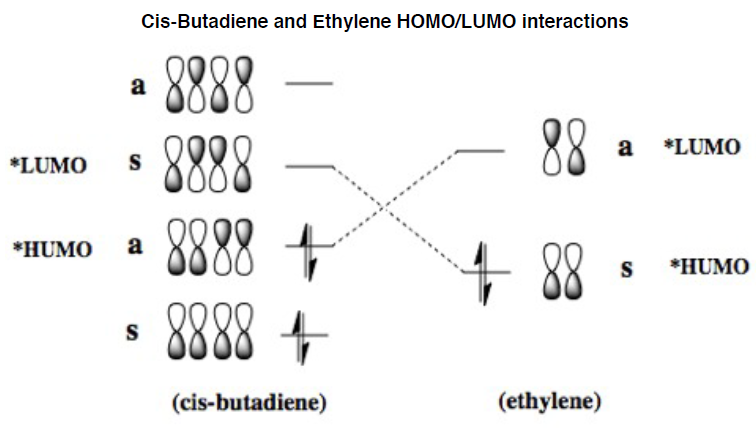
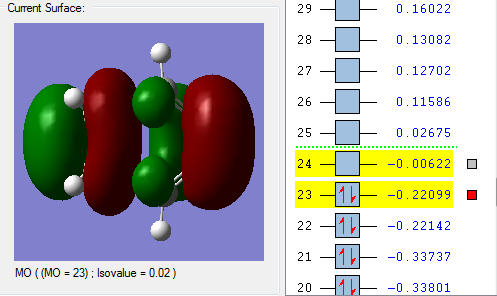
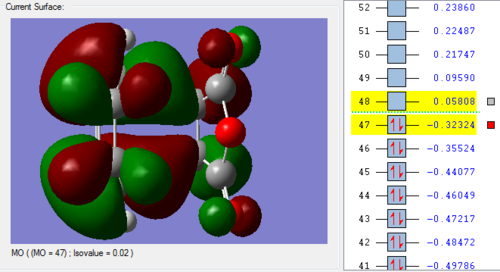
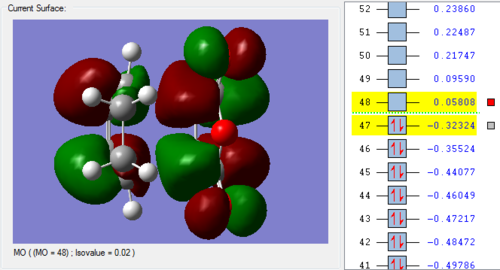
In the lowest energy conformation, there is totally symmetric orbital overlap between the p orbitals. The second-highest energy arrangement is having two pairs of p orbitals overlapping with 1 node. The next highest orbital conformation is the LUMO with just one pair of p orbitals overlapping with 2 nodes. The highest energy conformation has no favourable interactions between the p orbitals and has 3 nodes.The favourable overlap between the diene and dienophile is why it is important that butadiene is orientated in the cis position despite the trans conformation being more thermodynamically stable due to less electronic repulsion. Diels-Alder undergoes 'cis' [4+2] cycloaddition. As seen in the mechanism diagram previously, the transition state has a cyclic, 6-member form which suggests that bond forming/breaking is concerted in this reaction.
cis-Butadiene and ethylene MO interactions[1]
. In addition, the lowest energy orbital arrangement for butadiene would be totally symmetric with no nodes. There would be extensive delocalisation over the entire molecule. The highest energy orbital arrangement would be asymmetric with 3 nodes present. As bonds are being formed and broken at the same time, this mechanism is symmetric and synchronous.
Synchronous definition[1]
Studying regioselectivity of Diels-Alder
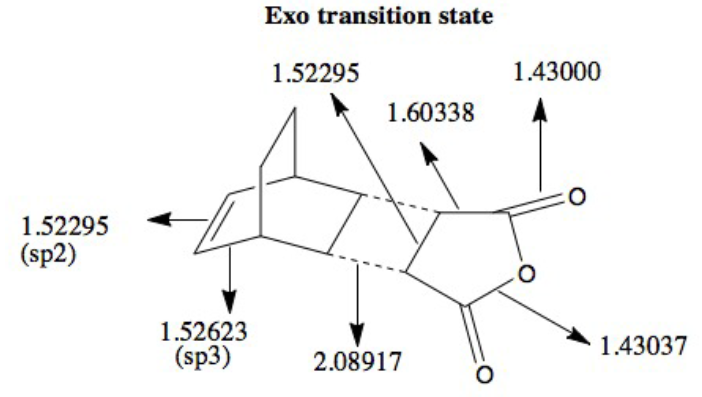
This last calculation was an opportunity to investigate the regioselectivity preferences for the Diels-Alder mechanism. This is simply the preference of one chemical bond direction when forming a product over an alternative method. The presence of EWG such as carbonyls on the dienophile (maleic anhydride) lower the HOMO/LUMO orbitals and result in a stronger interaction with the cyclohexa-1,3-diene orbitals.
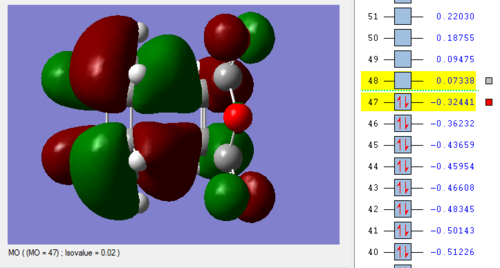
Link to optimised Endo structure:[15]
Vibration |
Job Convergence info:
Item Value Threshold Converged?
Maximum Force 0.000136 0.000450 YES
RMS Force 0.000026 0.000300 YES
Maximum Displacement 0.000705 0.001800 YES
RMS Displacement 0.000145 0.001200 YES
Predicted change in Energy=-9.332376D-08
Optimization completed.
-- Stationary point found.
Results Summary:
Link to frequency 'Endo' file: [16]
Thermochemistry values:
Sum of electronic and zero-point Energies= -605.414905 Sum of electronic and thermal Energies= -605.405479 Sum of electronic and thermal Enthalpies= -605.404535 Sum of electronic and thermal Free Energies= -605.450133
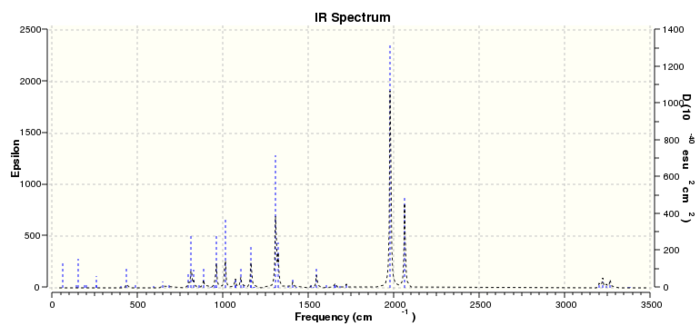
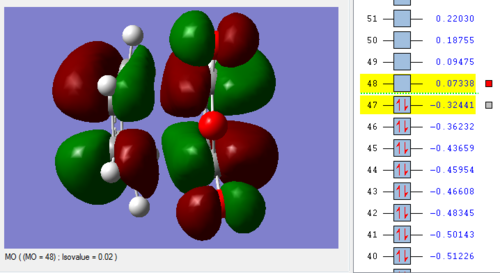
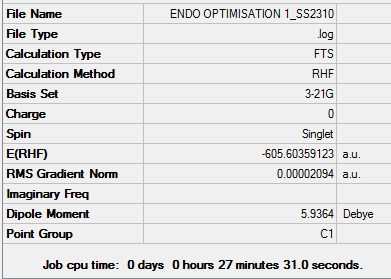
Link to Exo opt+freq structure:File:ENDO FREQ 1 SS2310.LOG
Vibration |
Job convergence info:
Item Value Threshold Converged?
Maximum Force 0.000108 0.000450 YES
RMS Force 0.000010 0.000300 YES
Maximum Displacement 0.000463 0.001800 YES
RMS Displacement 0.000119 0.001200 YES
Predicted change in Energy=-2.546980D-08
Optimization completed.
-- Stationary point found.
Results Summary:
Link to freq and opt 'Exo' conformer:File:ENDO FREQ 1 SS2310.LOG
Thermochemistry values:
Sum of electronic and zero-point Energies= -605.408138 Sum of electronic and thermal Energies= -605.398678 Sum of electronic and thermal Enthalpies= -605.397734 Sum of electronic and thermal Free Energies= -605.443689
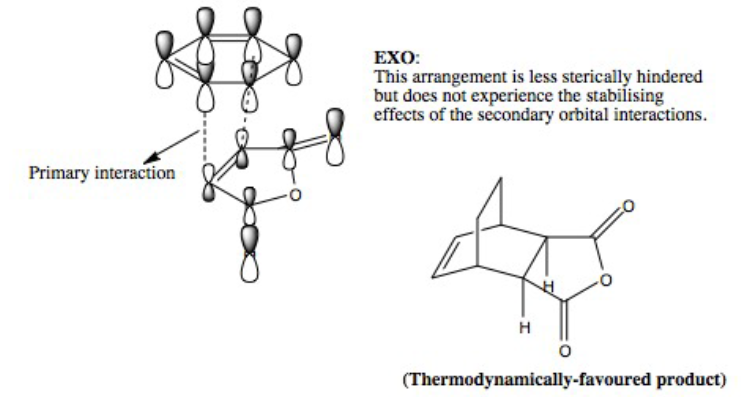
Endo vs Exo Summary:
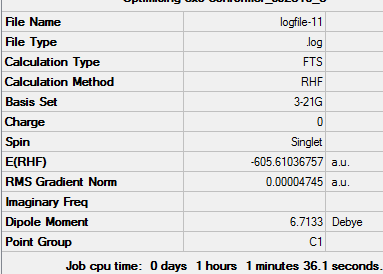
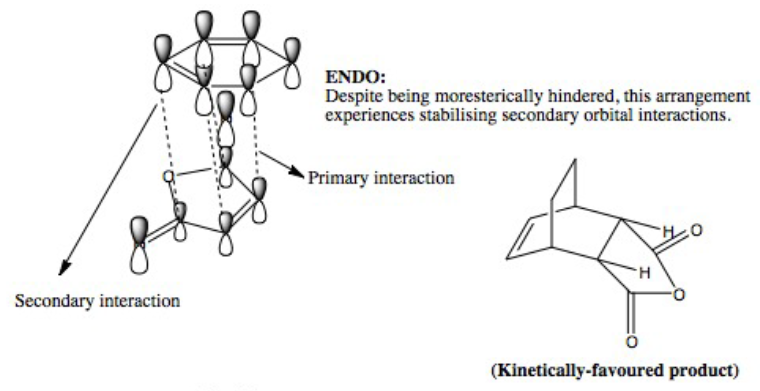
As the bonds are being formed and broken simultaneously, this is another example of a concerted, synchronous mechanism between an electron-rich diene (cyclohexa-1,3-diene) and an electron-poor dienophile with EWG (maleic anhydride). The orbitals fit the requirement that the there is favourable overlap due to matching symmetry and correct regioselective arrangement (cis-conformer for diene). The Endo product is the kinetically-favoured, major product of the reaction due to secondary orbital interactions between 4 carbon p orbitals despite the thermodynamically-favoured Exo product being sterically more favourable. This suggests that the secondary orbital interactions, when the EWG is below the diene, are sufficiently able to stabilised the structure and lower energy, even when compared to the Exo conformer. The transition energy values for both the Exo and Endo conformer are very close which suggests that the intermediate, transition structures are similar. However, the results of -605.61036757 and -605.60359123 hartree for the Endo and Exo respectively do not agree with the theory that the Endo will experience a lower energy. This may be due to an error in how the calculation was run in Gaussian, due to positioning of the original molecule.