Rep:OJA300793
Computational Chemistry: Physical
Tutorial: Cope Rearrangement
Reactants and Products
Anti-periplaner vs Gauche
In order to establish which conformer of 1,5-hexadiene is the most stable, optmization calcualtions have been carried out on both a typical Anti-periplanar (app) and gauche conformer.
1,5 Hexadiene Anti-peri planar Configuration Optimization
| 1,5 Hexadiene Optimization | ||
|---|---|---|
| File Name | OJA_1_5_hexadiene_optimization_3 | 
|
| File Type | .chk | |
| Calculation Type | FOPT | |
| Calculation Method | RHF | |
| Basis Set | 3-21G | |
| Charge | 0 | |
| Spin | Singlet | |
| Total Energy | -231.69253528 a.u. | |
| RMS Gradient Norm | 0.00001891 a.u. | |
| Imaginary Freq | ||
| Dipole Moment | 0.0000 Debye | |
| Point Group | ||
| Media:OJA_1_5_hexadiene_optimization_3.log | ||
Point Group: Ci
Energy: -231.69254 a.u.
1,5 Hexadiene Gauche Configuration Optimization
| 1,5 Hexadiene Gauche Optimization | ||
|---|---|---|
| File Name | OJA_1_5_hexadiene_optimization_gauche | 
|
| File Type | .chk | |
| Calculation Type | FOPT | |
| Calculation Method | RHF | |
| Basis Set | 3-21G | |
| Charge | 0 | |
| Spin | Singlet | |
| Total Energy | -231.69266122 a.u. | |
| RMS Gradient Norm | 0.00000702 a.u. | |
| Imaginary Freq | ||
| Dipole Moment | 0.3405 Debye | |
| Point Group | ||
| Media:OJA_1_5_hexadiene_optimization_gauche.log | ||
Point Group: C1
Energy: -231.69266
This data confirms that the Gauche conformer is more stable than the app one as it is lower in energy by 0.079kcal/mol. In comparison with the values given in the Tutorial Appendix it is clear that the reference conformer has been attained, and hence the lowest energy state of 1,5-Hexadiene, which has an energy of -231.69266 a.u.
When considering the Newman Projections, displayed below, of the two conformers it would be expected that the app conformer would be lower in energy due to a lack of steric interactions. However the distance between the two -CH=CH2 must be within the 'attractive' range of the two so by being gauche to one another there are in fact attractive forces between them, making the gauche conformer more stable. The through space bonding between the carbons on the two CH-CH2 groups have been tabulated below. The maximum attractive force between two carbons is at 3.2Å, and all bar one of these distances are greater than 3.2A meaning they are attractive. However some argue that the fact that the difference in energy of the conformers means it is impossible to establish a single one as the universal minima for the molecule[1]
| App | Gauche |
|---|---|
 |
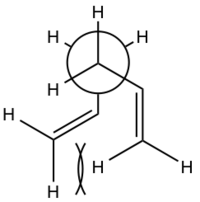
|
1,5 Hexadiene App Opt 631G(d)
| 1,5 Hexadiene App Optimization | |
|---|---|
| File Name | OJA_1_5_hexadiene_optimization_app_631Gd |
| File Type | .chk |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(D) |
| Charge | O |
| Spin | Singlet |
| Total Energy | -234.61170280 a.u. |
| RMS Gradient Norm | 0.00001326 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.0000 Debye |
| Point Group | |
| Media:OJA_1_5_hexadiene_optimization_app_631Gd.log | |
Point Group: Ci
By using a more advanced basis set a more accurate picture of the molecule can be obtained. The app has retained its symmetry, with both structures having Ci point group. To the naked eye the strucutres are the same but on closer inspection differences can be seen. For example corresponding bond lengths and angles are not identical across the two, as tabulated below. Although these differences are only small it demonstrates the affect of using a more advanced basis set.
| Basis Set | C1-C2 | C2-C3 | C3-C4 | C4-C5 | C5-C6 | C1-Ĉ2-C3 |
|---|---|---|---|---|---|---|
| 3-21 | 1.316Å | 1.509Å | 1.553Å | 1.509Å | 1.316Å | 124.8 |
| 6-31G* | 1.334Å | 1.504Å | 1.548Å | 1.504Å | 1.334Å | 125.3 |
In order to establish that a molecule has been optimised to a minima it is important to carry out a vibrational analysis on the optimised structure. If a minima has been achieved then all frequencies should be real, i.e none will be negative. A frequency analysis was carried out on 1,5-hexadiene using the 631G* structure.
1,5 Hexadiene App Frequency
| 1,5 Hexadiene App Frequency | IR Spectrum | |
|---|---|---|
| File Name | OJA_1_5_hexadiene_optimization_app_Freq | 
|
| File Type | .log | |
| Calculation Type | Freq | |
| Calculation Method | RB3LYP | |
| Basis Set | 6-31G(d) | |
| Charge | 0 | |
| Spin | Singlet | |
| Total Energy | -234.61171021 a.u. | |
| RMS Gradient Norm | 0.00001497 a.u. | |
| Imaginary Freq | 0 | |
| Dipole Moment | 0.0000 Debye | |
| Point Group | CI | |
| Media:OJA_1_5_hexadiene_optimization_app_Freq.log | ||
Point Group: Ci
In the table above the IR spectrum can be seen, which shows all real frequencies only, therefore this structure is a minima. Below the Thermochemistry data for the molecule has been extracted from the calculation file.
Thermochemistry
Sum of electronic and zero-point Energies= -234.469204 Sum of electronic and thermal Energies= -234.461857 Sum of electronic and thermal Enthalpies= -234.460913 Sum of electronic and thermal Free Energies= -234.500778
Transition States
In the Cope Rearrangement there are two transition states, the chair and the boat. In this exercies both will be optimised and evaluated in order to build a better picture of what happens in the reaction.
Chair Transition State
| Chair TS Optimization & Frequency | Vibration | |
|---|---|---|
| File Name | OJA_Chair_TS_Guess_1 | 
|
| File Type | .chk | |
| Calculation Type | FREQ | |
| Calculation Method | RHF | |
| Basis Set | 3-21G | |
| Charge | 0 | |
| Spin | Singlet | |
| Total Energy | -231.61932246 a.u. | |
| RMS Gradient Norm | 0.00000727 a.u. | |
| Imaginary Freq | 1 | |
| Dipole Moment | 0.0003 Debye | |
| Point Group | Imaginary Frequency is at -817.94 cm-1 | |
| Media:OJA_Chair_TS_Guess_1.log | ||
By combining optimisation and frequency only 1 calculation needs to be run through Gaussian. As can be seen by the animation above this imaginary frequency firstly shows that this structure is a minimum and secondly that this is how the transition state develops towards the product.
There is more than one way of optmising a transition state and the Bond Freeze method has been used below.
Chair Transition State Bond Freeze Opt
| Chair TS Bond Freeze Optimization | ||
|---|---|---|
| File Name | OJA_Chair_TS_bond_freeze_2_berny | 
|
| File Type | .log | |
| Calculation Type | FTS | |
| Calculation Method | RHF | |
| Basis Set | 3-21G | |
| Charge | 0 | |
| Spin | Singlet | |
| E(RHF) | -231.61932239 a.u. | |
| RMS Gradient Norm | 0.00002349 a.u. | |
| Imaginary Freq | ||
| Dipole Moment | 0.0005 Debye | |
| Point Group | C1 | |
| Media:OJA_Chair_TS_bond_freeze_2_berny.log | ||
On direct inspection the two structures found look very similar and have similar energies. A table below shows the bond breaking/forming bond lengths in the molecules. As the values are the same it shows that both of these methods are valid when optimising the transition state.
| Structure | Bond Forming | Bond Breaking |
|---|---|---|
| Opt&Freq | 2.021 Å | 1.389 Å |
| Bond Freeze | 2.021 Å | 1.389 Å |
| Carbon | Bond Distance | |
|---|---|---|
| Opt & Freq | Bond Freeze | |
| C3 - C14 | 2.02051Å | 2.02064Å |
| C6 - C11 | 2.02051Å | 2.02064Å |
Boat Transition State
In order to optimise the boat transition structure it must be in a geometry similar to the optimised structure before the calculation is carried out. When attempted in a linear chain form the calculation failed, and only ran when the molecules were manipulated to resemble a 'C' did the calculation run.
| Boat TS Optimization & Frequency | Vibration | |
|---|---|---|
| File Name | OJA_Boat_TS_Opt_Freq_Finally | 
|
| File Type | .chk | |
| Calculation Type | FREQ | |
| Calculation Method | RHF | |
| Basis Set | 3-21G | |
| Charge | 0 | |
| Spin | Singlet | |
| Total Energy | -231.60280247 a.u. | |
| RMS Gradient Norm | 0.00001668 a.u. | |
| Imaginary Freq | 1 | |
| Dipole Moment | 0.1583 Debye | |
| Point Group | Imaginary Frequency is at -840.03 cm-1 | |
| Media:OJA_Boat_TS_Opt_Freq_Finally.log | ||
Chair IRC
| Chair IRC | |
|---|---|
| File Namecell | OJA_Chair_TS_IRC_4 |
| File Typecell | .log |
| Calculation Type cell | IRC |
| Calculation Methodcell | |
| Basis Setcell | |
| Charge cell | 0 |
| Spincell | Singlet |
| E(RHF)cell | -231.61932246 a.u. |
| RMS Gradient Normcell | 0.00000728 a.u. |
| Imaginary Freqcell | |
| Dipole Momentcell | 0.0003 Debye |
| Point Groupcell | C1 |
| Media:OJA_Chair_TS_IRC_4.log | |
On the link below a video of the IRC can be found.
File:OJA Chair TS TO PRODUCT.m4v
However the IRC doesn't confirm which final structure has been formed so an optimisation to a minimum was carried out on the final structure given.
| IRC Structure Optimization | ||
|---|---|---|
| File Name | OJA_Chair_IRC_Final_Structure_Opt | 
|
| File Type | .chk | |
| Calculation Type | FOPT | |
| Calculation Method | RHF | |
| Basis Set | 3-21G | |
| Charge | 0 | |
| Spin | Singlet | |
| E(RHF) | -231.69166702 a.u. | |
| RMS Gradient Norm | 0.00000475 a.u. | |
| Imaginary Freq | ||
| Dipole Moment | 0.3806 Debye | |
| Point Group | C1 | |
| Media:OJA_Chair_IRC_Final_Structure_Opt.log | ||
From this it can be seen that Gauche conformer has been formed, and by comparing the energies given in Appedix 1 on the tutorial sheet is between Gauche 2 and Gauche 4. Although this is not the most accurate way of establishing the conformer it is quick and is good to use as an approximation.
Reoptimization of Chair And Boat
| Chair TS Reoptimization & Frequency | |
|---|---|
| File Name | OJA_Chair_TS_ReOpt_freq |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(D) |
| Charge | O |
| Spin | Singlet |
| E(RB3LYP) | -234.55698295 a.u. |
| RMS Gradient Norm | 0.00002879 a.u. |
| Imaginary Freq | 1 |
| Dipole Moment | 0.0003 Debye |
| Point Group | C1 |
| Media:OJA_Chair_TS_ReOpt_freq.log | |
| Boat TS Reoptimization & Frequency | |
|---|---|
| File Name | OJA_Boat_TS_ReOpt_freq |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(D) |
| Charge | O |
| Spin | Singlet |
| E(RB3LYP) | -234.54309366 a.u. |
| RMS Gradient Norm | 0.00008351 a.u. |
| Imaginary Freq | 1 |
| Dipole Moment | 0.0612 Debye |
| Point Group | C1 |
| Media:OJA_Boat_TS_ReOpt_freq.log | |
Summary Table
Below is a compilation of all the Energy data collected across the calculations run in this exercise for the Reactants and Char and Boat Transition structures for the Cope Rearrangement. These are in line with those given in Appendix 2 of the Tutorial.
| Molecule | HF/3-21G | B3LYP/6-31G* | ||||
|---|---|---|---|---|---|---|
| Electronic Energy | Sum of Electronic and Zero-Point Energies (0K) | Sum of Electronic and Thermal Energies (298.15K) | Electronic Energy | Sum of Electronic and Zero-Point Energies (0K) | Sum of Electronic and Thermal Energies (298.15K)
| |
| Reactant (App) | -231.69253528 | -231.539539 | -231.532565 | -234.61171021 | -234.469204 | -234.461857 |
| Chair TS | -231.61932246 | -231.466701 | -231.461341 | -234.55698295 | -234.414932 | -234.409011 |
| Boat TS | -231.60280247 | -231.450930 | -231.445301 | -234.54309366 | -234.402347 | -234.396012 |
| Molecule | HF/3-21G | B3LYP/6-31G* | ||
|---|---|---|---|---|
| (0K) | (298.15K) | (0K) | (298.15K) | |
| ΔE (Chair) | 45.71 | 44.69 | 34.06 | 33.16 |
| ΔE (Boat) | 55.60 | 54.76 | 41.95 | 41.32 |
Diels Alder Reaction
Butadiene
| Butadiene Optimization & Frequency | |
|---|---|
| File Name | OJA_Butadiene_Opt_Freq |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -155.98595614 a.u. |
| RMS Gradient Normc | 0.00002726 a.u. |
| Imaginary Freq | 1 |
| Dipole Moment | 0.0854 Debye |
| Point Group | CS |
| Media:OJA_Butadiene_Opt_Freq.log | |
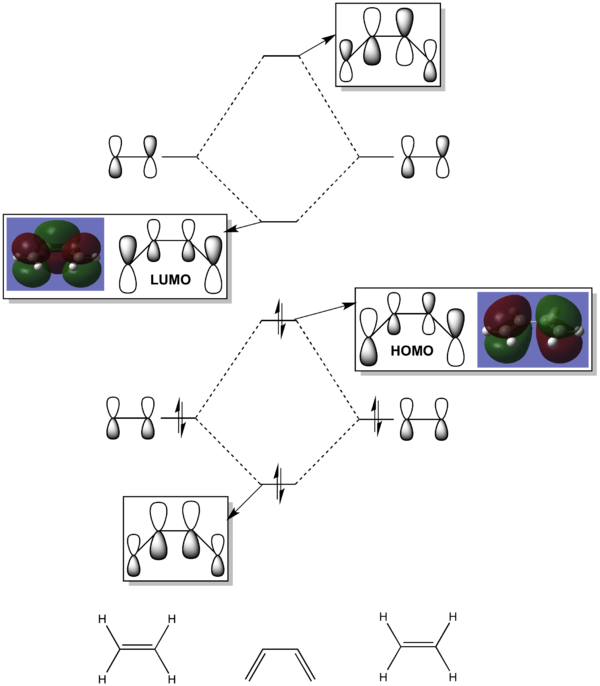
From this structure the HOMO and LUMO have been plotted, as shown below.
| Butadiene Molecular Orbitals | |
|---|---|
 |
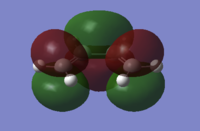 |
| HOMO, asymmetric with respect to the plane, a | LUMO, symmetric with respect to the plane, s |
A sketch of the MOs has been made for ease of interpretation. The π orbitals are very distinct in the Gaussian MOs and the orbital contributions can clearly be seen; carbons 2 and 3 contribute less than 1 and 4 hence their smaller orbitals.
Transition State
The transition state for a Diels Alder reaction between Butadiene and Ethene was constructed and optimised using TS(Berny) and calculating the Force Constants Once. This method was used after failing to run a TS(QST2) calculation due to a lack of similarity of structures submitted against the actual transition state minima.
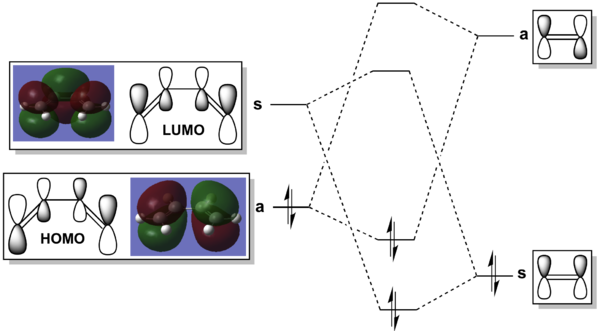
A frontier orbital molecular orbital diagram of butadiene and ethene can be seen below. This transiotn is allowed due to the mathcing orbital symmetry for Ethene(LUMO)-Butadiene(HOMO) and Ethene(HOMO)-Butadiene(LUMO) denoted by an s (symmetric) and an a (asymmetric) with respect to the plane on the MO diagram.
| Diels Alder TS Opt&Freq | ||
|---|---|---|
| File Name | OJA_DA_TS_Guess_8 | 
|
| File Type | .chk | |
| Calculation Type | FREQ | |
| Calculation Method | RB3LYP | |
| Basis Set | 6-31G(d) | |
| Charge | 0 | |
| Spin | Singlet | |
| E(RB3LYP) | -234.54389655 a.u. | |
| RMS Gradient Normc | 0.00000182 a.u. | |
| Imaginary Freq | 1 | |
| Dipole Moment | 0.3942 Debye | |
| Point Group | ||
| Media:OJA_DA_TS_Guess_8.log | ||
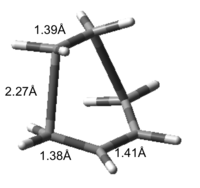
This structure can be confirmed as a transition state due to the presence of a single imaginary frequency at -525cm-1 which matches the proposed mechanism of the Diels Alder Reaction. The formation of the two bonds is synchronous, as shown by the vibration, however the lowest 'real' frequency of the molecule is an asynchronous twist of the ethene molecule, giving an obvious distinction between the 'reacting' vibration and the real one.
The partially formed C-C σ bonds in the transition state have a length of 2.27Å, which is in accordance with literature[2] values found. This value is larger than both sp3 and sp2 carbon bonds (1.54Å and 1.47Å respectively) but is smaller than the sum of Carbons van der Waals Radii (3.4Å, 1.7Å per carbon) indicating that a bond is in the process of forming and this inter atomic distance will contract as shown in the vibration above.
The bond lengths of the other carbon bonds can be seen in Figure 1.
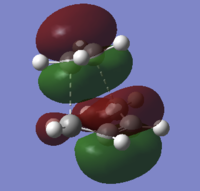
On inspection of the HOMO of the Transition State it can be seen to be made up of a mix of π and sp3 orbitals as certain π bonds break and the orbitals hybridise to sp3 orbitals. The fact that the symetrical homo-π orbitals of ethene are seemingly intact in the transtion state it can be specualted the the transition state HOMO is the combination Ethene(HOMO)-Butadiene(LUMO). From the MO diagram at the beginning of this section, this combination does not produce the HOMO of cyclohexene indicating that this orbital once reacted will lower itself in energy below the Ethene(LUMO)-Butadiene(HOMO) combination. The LUMO, as predicted in the MO is a combination of Ethene(HOMO)-Butadiene(LUMO) but inverted. A sketch along with the calculated MO diagrams can be found in the table below.
| Transition State Molecular Orbitals | |
|---|---|
 |
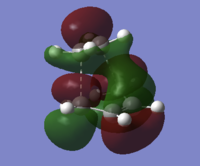 |
| HOMO, s | LUMO, s |
 |
 |
Cyclohexa-1,3-diene & Maleic Anhydride
In order to determine the transition states in the Diels Alder reaction between Cyclohexa-1,3-diene and Maleic Anhydride, the reactants and products were optimised for comparative purposes. The optimised reactant structures were then used in order to assemble the 'guess' transition states before they were optimised.
| Cyclohexa-1,3-diene Optimization | Maleic Anhydride Optimization | |
|---|---|---|
| File Name | OJA_Cyclohexa13_Opt | OJA_MaleicAnhydride_Opt |
| File Type | .chk | .chk |
| Calculation Type | FOPT | FOPT |
| Calculation Method | RB3LYP | RB3LYP |
| Basis Set | 6-31G(D) | 6-31G(D) |
| Charge | 0 | 0 |
| Spin | Singlet | Singlet |
| Total Energy | -233.41891004 a.u. | -379.28954412 a.u. |
| RMS Gradient Norm | 0.00013317 a.u. | 0.00011730 a.u. |
| Imaginary Freq | ||
| Dipole Moment | 0.3785 Debye | 4.0749 Debye |
| Point Group | ||
| log File: | Media:OJA_Cyclohexa13_Opt.log | Media:OJA_MaleicAnhydride_Opt.log |
| Diels Alder Endo Product Optimization | Diels Alder Exo Product Optimization | |
|---|---|---|
| File Name | OJA_DA_Pr_Exo | OJA_DA_Pr_Endo |
| File Type | .fch | .fch |
| Calculation Type | FOPT | FOPT |
| Calculation Method | RB3LYP | RB3LYP |
| Basis Set | 6-31G(D) | 6-31G(D) |
| Charge | 0 | 0 |
| Spin | Singlet | Singlet |
| Total Energy | -612.75578531 a.u. | -612.75829018 a.u. |
| RMS Gradient Norm | 0.00003162 a.u. | 0.00001678 a.u. |
| Imaginary Freq | ||
| Dipole Moment | 4.7592 Debye | 5.0202 Debye |
| Point Group | ||
| D-Space | DOI:10042/25964 | DOI:10042/25963 |
The transition states were optimised to TS(Berny) with force constants calculated once, as in the Diels Alder reaction between Butadiene and Ethene. The Imaginary Frequencies can be seen below the summary table and show the breaking and forming of the bonds in the transition of the reaction.
| Diels Alder TS Exo Opt&Freq | Diels Alder TS Endo Opt&Freq | |
|---|---|---|
| File Name | OJA_DA_TS_Exo_2 | OJA_DA_TS_Endo_1 |
| File Type | .fch | .fch |
| Calculation Type | FREQ | FREQ |
| Calculation Method | RB3LYP | RB3LYP |
| Basis Set | 6-31G(D) | 6-31G(D) |
| Charge | 0 | 0 |
| Spin | Singlet | Singlet |
| Total Energy | -612.67931094 a.u. | -612.68339675 a.u. |
| RMS Gradient Norm | 0.00001057 a.u. | 0.00001893 a.u. |
| Imaginary Freq | 1 | 1 |
| Dipole Moment | 5.5504 Debye | 6.1143 Debye |
| Point Group | ||
| D-Space | DOI:10042/25967 | DOI:10042/25968 |
| Imaginary Frequencies | |
|---|---|
 |

|
| Exo, -448cm-1 | Endo, -447cm-1 |

The comparitve energy to the reactants has been tabulated with the exo transition state requiring a greater amount of energy. This reaction is kinetically controlled meaning that the endo product will be favoured as the major product. This is well represented by the reaction coordinate in Figure 2, the blue line is the Endo formation and the red line is the exo formation.
| Isomer | ΔE |
|---|---|
| Exo | 18.32 kcal/mol |
| Endo | 15.75 kcal/mol |
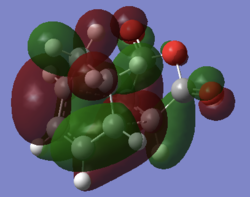
Although there is only a relatively small difference in activation energy for the two transition states there will be a large difference in products formed due to the exponential relationship between energy and rate in the Arrhenius equation. The lower activation energy for the Endo transition state can be put down to secondary orbital interactions. This is where the carbon π orbital on the Anhydride -C=O interacts with the carbon π orbital in the diene in a positive way to stabilise the whole molecule. There is a through space bond distance of 3Å, which is less than the sum of the van der waals radii of two carbon atoms, meaning that the two orbitals are close enough to interact. This stabilisation is lost in the exo structure as the Anhydride π orbital cannot interact with the sp3 orbital of the carbon it is opposite due to a lack of matching orbital symmetry despite being within the sum of the van der Waals raddii. This indicates that this interaction is a potentially repulsive one, hence the higher activation energy required to reach this state.
On inspection of the HOMOs of the two transition states the SOI can definitely be seen in the endo structure, with a mass of electron density centred between the double bond in the diene and the maleic anhydride
| Exo | Endo |
|---|---|
 |
 |
| a | a |
| Bond Lengths | |
|---|---|
 |

|
| Exo | Endo |
References
- ↑ DOI:10.1080/00268970110081412 An analysis of the conformers of 1,5-hexadiene, BRANDON G. ROCQUE, JASON M. GONZALES & HENRY F. SCHAEFER III
- ↑ Dynamics, transition states, and timing of bond formation in Diels–Alder reactions Kersey Blacka,Peng Liub,Lai Xub,Charles Doubleday, Kendall N. Houkb http://www.pnas.org/content/109/32/12860.full