Rep:Namespace:jr4073PROJECT
Modelling Techniques & Calculations
The computational models may be built from ChemDraw structural drawings, which convert to 3D structures in Chem3D, or by direct modelling in GaussView. Optimisations may be run by MM2 (molecular mechanics) or PM6 (semi-empirical molecular orbital theory) in Chem3D. The principale method here is the use of Gaussian to run molecular orbital-based quantum calculations - numerically solving the Schroedinger equation - giving a more accurate result, and semi-empirical (AM1) calculations to show MOs.
The Diels-Alder Cycloaddition
A Diels Alder reaction is a pericyclic reaction between a diene and a dieneophile, the π orbitals of the two species interacting to form new σ bonds. For such a reaction to be allowed the HOMO of one reactant must interact with the LUMO of the other (forming two new MOs, one bonding and one anti-bonding). Furthermore, for a reaction there must be good orbital overlap; differing symmetry makes this impossible and the reaction forbidden.
The addition of substituents (with π orbitals) to the dieneophile stabilises the regiochemistry of the addition by interacting with the new double bond on the product (known as secondary orbital effects).
The transition structures for Diels-Alder cycloadditions - with both substituted and unsubstituted dieneophiles - are determined, considering the MOs also, to predicted reactivity. Under kinetic control the product with the lower energy transition state dominates, as illustrated below.
Prototypical Diels-Alder cycloaddition: ethylene + cis-butadiene
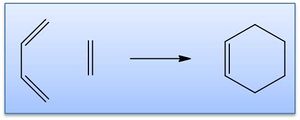
The reaction scheme between ethylene and butadiene to give cyclohexene via a Diels-Alder cycloaddition is shown right. The way in which the reactants come together in the transition state is governed by π orbital interactions, principally the HOMO and LUMO of each. In this case there is a [4s + 2s] addition (this reflects the number of π orbitals present). The MO interactions are considered in more detail below, with the transition states of such cycloadditions to predict reactivity.
MO analysis of cis-butadiene
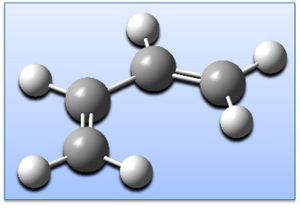
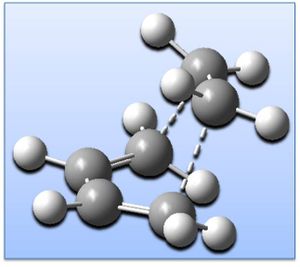
Cis-butadiene has been geometry optimised (AM1 level) giving the structure shown right (click to enlarge & click button for 3D JMol). Optimisation is confirmed in the .log output file and by frequency (vibrational) analysis - there are no imaginary frequencies and thus a global minima has been achieved:
Optimization completed.
-- Stationary point found.
Low frequencies --- -39.4293 -0.3433 -0.2361 -0.1031 -0.0005 0.9267 Low frequencies --- 2.5946 312.4390 485.2250
The thermal energies of the system are noted:
Sum of electronic and zero-point Energies= 0.134552 Sum of electronic and thermal Energies= 0.138573 Sum of electronic and thermal Enthalpies= 0.139517 Sum of electronic and thermal Free Energies= 0.109172
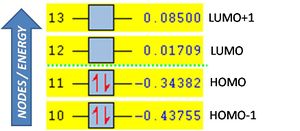
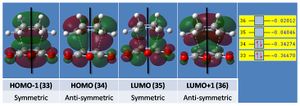
The HOMO and LUMO are shown below with their symmetry relative to a vertical plane (sketched).

Notably the HOMO is anti-symmetric and the LUMO symmetric, and the number of nodes increases with the energy of the MO (energies are shown right). The optimised molecule is fully planar with a dihedral angle of 0deg (C-C-C-C).
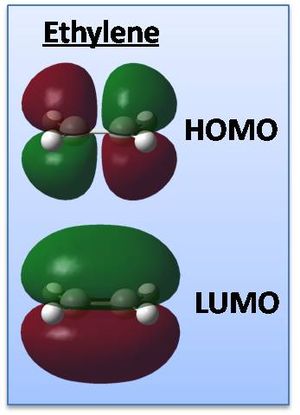
The MOs of ethylene have also been computed in the same manner (shown right), the HOMO being symmetric and the LUMO anti-symmetric this time though, with no nodal planes in the HOMO and one in the LUMO.
Optimising the transition structures
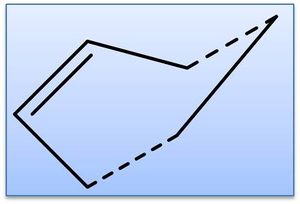
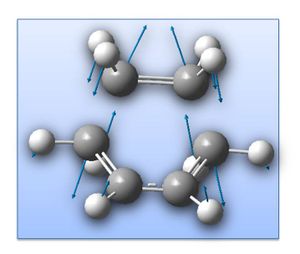

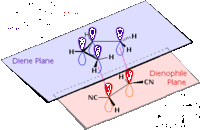
The transition state for the reaction between ethylene and cis-butadiene is known to have an envelope type structure to maximise the π orbital overlap between the reactants. The transition state structure has been optimised from an initial guess, shown right (AM1 level; TS (Berny)). The guess structure was achieved after using through space fragment separations of 2.2a.u. and 1.5a.u., which resulted in returning separate reactants and the product respectively - adjusting to 2.00a.u. allowed for a transition state. Simultaneous frequency analysis confirms this to be a transition state by the presence of an imaginary vibration:
Low frequencies --- -956.1133 -3.7882 -2.8348 -0.4038 -0.0031 0.0485 Low frequencies --- 0.0842 147.2135 246.6165
The thermal energies of the system are noted:
Sum of electronic and zero-point Energies= 0.253277 Sum of electronic and thermal Energies= 0.259454 Sum of electronic and thermal Enthalpies= 0.260398 Sum of electronic and thermal Free Energies= 0.224017
Animation of the imaginary vibration corresponds to the pericyclic reaction as the two fragments come together (shown right - click to enlarge).
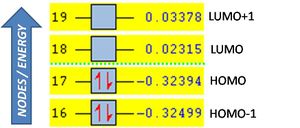
The principal MOs of the transition state and their symmetry are described below, their relative energies being plotted right.
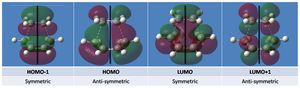
Notably the HOMO has three nodes creating six clouds of electron density in an antisymmetric array about the plane shown.
Discussion
Typically sp3 bonded carbon has a C-C bondlength of 1.54Å, and sp2 carbon a C=C bondlength of 1.34Å.[1] The observed bond lengths are intermittant between the two, indicative of a transition state with delocalised bonding. In addition, carbon has an atomic van der Waals radius of 1.70Å.[2] thus non-bonded carbons must be 3.4Å apart, the through space separation is 2.12Å confirming a transition structure with partial bonds.
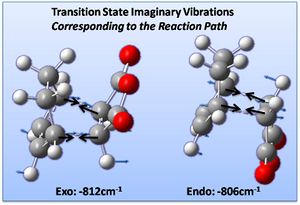
The vibration corresponding to the reaction path at the transition state is shown right - as discussed above - representing synchronous formation of the two bonds. Below this the lowest real vibration is observed, a twisting of the dieneophile fragment over the diene, this is unlikely to correspond to a reaction path, as it does not bring the fragments closer together.
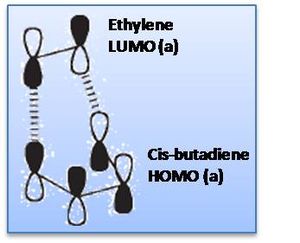
The HOMO of the transition structure is shown above, being anti-symmetric (a); the HOMO of cis-butadiene interacts with the LUMO of ethylene to form the transition state HOMO, as shown below.
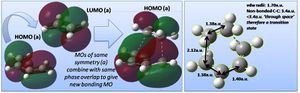
Notably the LUMO of the transition state is formed by the combination of the ethylene HOMO and cis-butadiene LUMO (both symmetric, s); the ethylene HOMO and LUMO are of course the pi and pi* bonding and anti-bonding MOs. The representation of HOMO-LUMO interaction in terms of AOs is shown right.
Regioselectivity of the Diels-Alder reaction
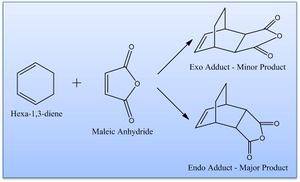
It is known that cyclohexa-1,3-diene reacts with maleic anhydride giving the endo adduct as the major product - this is supposed to be a result of kinetic control and the exo transition state being higher in energy. The reaction is shown right.
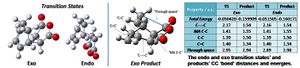
The transition structures leading to both products are shown below, with their corresponding energies below and C-C 'bond' distances tabulated. These were determined as above, by the TS (Berny) optimisation and frequency method (semi-empirical AM1 level; opt=noeigen, always calculate force constants), from an initial guess structure, based on the literature.
The energies of the endo transition state are noted:
Sum of electronic and zero-point Energies= 0.133494 Sum of electronic and thermal Energies= 0.143683 Sum of electronic and thermal Enthalpies= 0.144628 Sum of electronic and thermal Free Energies= 0.097351
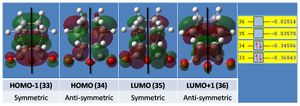
And the principal MOs illustrated below.
And of the exo transition state:
Sum of electronic and zero-point Energies= 0.134881 Sum of electronic and thermal Energies= 0.144882 Sum of electronic and thermal Enthalpies= 0.145827 Sum of electronic and thermal Free Energies= 0.099117
Again the principal MOs are again shown below.
The sum of free energies (and all other thermal chemistry properties listed) for the endo transition state are lower than for the exo; the C-C 'bond' distances are highly similar in both cases, as is the general shape of the MOs, however these are much lower again for the endo. This confirms that under kinetic control the endo adduct will dominate as the precursing transition state is reached sooner.
Discussion
The relative energies of the transition structures (cyclohexa-1,3-diene reaction with maleic anhydride) are tabulated above (exo: -0.050420a.u.; endo: -0.051505a.u.), the endo being lower in energy and more stable by 0.68 kcal/mol. This confirms that under kinetic control the endo transition state will be reached first and the endo adduct dominates. The exo form is more strained most likely as a result of steric repulsion between the oxygen atoms on the carbonyl groups and the hydrogens on the bridging sp3 carbons, which have a through space separation of 2.69a.u.; this is in comparison to the endo form where the carbonyl oxygens are now in steric repulsion with a hydrogen on sp2 carbon (which has a planar configuration, increasing the O-H separation), the through space distance increasing to 3.31a.u.. In addition, the HOMO of the exo system has a node not present in the endo, as shown below; the endo benefits from a more extensive electron cloud conferring greater stability to the structure.
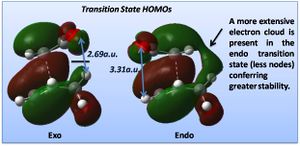
This is in part due to the pi bonding of the two fragments being on the same face of the molecule in the endo transition state, leaving sigma bonding MOs on the other face that can interact together (secondary orbital overlap). In the exo structure the pi and sigma bonding MOs cannot interact (they are now opposite each other), leading to a nodal plane.
Like the reaction of ethylene and cis-butadiene, the reaction is likely to proceed via donation from the HOMO of the diene to the LUMO of the dieneophile (maleic anhydride); this is further enhanced by the electron withdrawing substituents on the diene, creating a dipole to pull electron density towards itself.
Vibrational analysis confirms the optimised structures to be transition states (by the presence of a negative [imaginary] vibration indicating a local [not global] minima). Animating this vibration for both structures shows correspondence to the reaction path to the products: as shown below.
Error analysis...
Calculated energies are accurate to ~10kJ/mol; dipole moments are accurate to 0.01 Debye, and frequencies reported to 1cm-1 (typically 10% error), likewise intensities (infrared) are given as whole numbers; bond distances are accurate to 0.01a.u. and angles to 0.1deg.
Notably energies between molecules should only be considered on a relative (kJ/mol; not absolute!) level; absolute values are noted for reproducibility only (a.u.), and consistency is paramount for comparison (basis set, method, details).
References
- ↑ Atkins, P., and de Paula, J., Physical Chemistry, (8th Ed.), Oxford: Oxford University Press, 2006
- ↑ Cambridge Crystallographic Data Centre: http://www.ccdc.cam.ac.uk/products/csd/radii/table.php4
External links
- [Main Page: https://www.ch.ic.ac.uk/wiki/index.php/Namespace:jr4073]
- [Michigan State University - Reactions of Alkenes: http://www.cem.msu.edu/~reusch/VirtualText/addene2.htm]
- [Chemistry SCAN Link: https://scanweb.cc.imperial.ac.uk/uportal2/]
- [Illustration of vibrational mode categories: http://en.wikipedia.org/wiki/Infrared_spectroscopy]
- [Online symmetry point group flow chart: http://capsicum.me.utexas.edu/ChE386K/Images/point_group_flowchart_shriver.jpg]
