Rep:Module 3 part 2:mh108 3apr9tru
The Diels Alder Cycloaddition
Molecular Orbitals of butadiene
Method
Butadiene was simulated by Gaussview03 and optimised at B3LYP/6-31G level by Gaussian03W. The molecular orbitals were then calculated at the same level of calculation to ensure accuracy. Finally the MO symmetry was assigned for further comparison with the Diels Alder Cycloaddition transtion state.
Result
| Table 6 | cis-Butadiene OPT |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G |
| E(RB3LYP) | -155.95382512 a.u. |
| RMS Gradient Norm | 0.00001691 a.u. |
| Dipole Moment | 0.0941 Debye |
| Point Group | C2V |
The optimisation summary can be viewed in table 6 on the right. The important result of this calculation are the symmetries of highest occupied and lowest unoccupied molecular orbitals (HOMO and LUMO). The HOMO is a bonding orbital, which is antisymmetric with respect to xz and yz plane and symmetrical with respect to xy plane. The LUMO is still a bonding orbital, although it is on the border line and can be considered non-bonding. It is symmetrical with respect to xy and xz planes but anti-symmetrical with respect to yz plane.
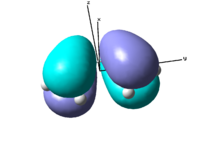 |
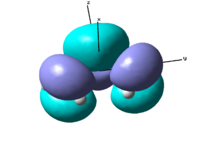 |
Discussion
Ethene the dienophile in the Diels Alder Cycloaddition under consideration approaches butadiene from front of the top or bottom face, parallel to xy and yz plane, where the HOMO-LUMO interactions can occur, thus the symmetry with respect to these planes are not relevant. However the perpendicular interaction with respect to xz plane stresses the importance of MO reflectional symmetry with respect to this plane.
In conclusion, even though both HOMO and LUMO of butadiene are symmetrical and antisymmetrical depending on the plane under consideration, it is only the symmetry with respect to xz plane that is relevant thus the HOMO is anti-symmetrical and LUMO symmetrical. Furthermore the HOMO and LUMO of ethene have complementary symmetry with respect to the xz plane allowing for interactions.
Prototype Transition State in Diels Alder Cycloaddition
Method

| Table 7 | Diels Alder TS |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -234.49456035 a.u. |
| RMS Gradient Norm | 0.00002031 a.u. |
| Imaginary Freq | 1 |
| Dipole Moment | 0.4506 Debye |
| Point Group | C1 |
Assuming envelope like structure of the transition state in Diels Alder Cycloaddition of butadiene with ethene as shown in fig. 1, where the butadiene fragment and ethene fragment were frozen 2.14A apart and optimised using Mod-redundant at HF/3-31G level using Gaussian03W. After the optimisation the bonds were unfrozen and derivated to reach a transition state at the same level of theory. Finally the pre-optimised transition structure was optimised at B3LYP/6-31G level and molecular orbitals computed at the same level of theory.
Results
The transition state was shown to have an envelope like structure with a bond angle of 102.2°±0.1 between the former ethene fragment and butadiene fragment. The newly forming C-C σ bonds have bond length of 2.26A and an imaginary frequency of -534cm-1. The transition structure can be seen in Jmol in fig.1 with the optimisation summary in table 7.
The imaginary vibration is a synchronous stretching/compressing vibration corresponding to the concerted formation of the two new C-C σ bonds. On the other hand the first real vibration is a an asynchronous bending vibration corresponding to twisting of the ethene fragment with respect to the reaction plane.
 |
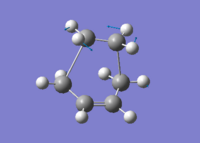 |
 |
 |
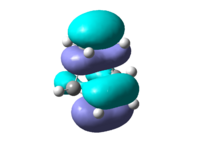
The HOMO and LUMO of ethene are shown for illustration of the MOs participating in the reaction. The HOMO is a symmetrical orbital and the LUMO antisymmetrical with respect to the xz plane. Finally the resulting HOMO of the transition state is symmetrical MOs.
Discussion
The sp3, sp2 bond lengths for carbon are 1.53A and 1.34A respectively[1] both much shorter than the two forming σ C-C bonds in the transition state. However the newly forming bonds are shorter than the sum of Van der Waals radii of two carbon atoms, 3.40A.<ref name="Bond lengths"> Furthermore the bond lengths of the original C=C in the starting material have prolonged to 1.39A and the single bond contracted to 1.41A indicating shifting of the electron density in the transition state compared to the starting material. It can be concluded that the structure shown is indeed a transition state, where sp2 bonds are broken and new sp3 bonds formed in a concerted fashion as similar bonds have similar lengths.
Similar conclusion can be made from the MO mixing. The HOMO of ethene is mixing with the LUMO of butadiene resulting in an orbital with even electronic distribution in the transition state i.e comparable bond lengths for originaly similar bonds. The mixing, and thus the reaction is allowed, due to similar molecular orbital symmetry. If the symmetry waas opposite the reaction could not proceed due to selection rules.
