Rep:Module 1:mh108 3apr9tru
Introduction to Molecular Mechanics
Molecular Mechanism, further referred as MM, is an approximate method for quantifying molecular strain and steric effects by systematically varying the individual atomic positions within a molecule in order to achieve the lowest possible energy i.e. the most stable state. The energy of the molecule is based on five summations:
- Diatomic bond stretches
- Triatomic bond angle deformations
- Tetra-atomic bond torstions
- Non-bonded Van der Waals repulsions
- Electrostatic attractions of individual bond dipoles [[1]]
However it should be noted that energy provided by MM is only a comparative value not a thermodynamic property thus can be used to evaluate relative stability of a set of isomers but cannot be used to predict for example the free energy of a reaction. For more information visit: [Molecular Mechanics]
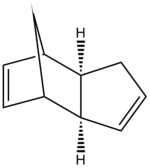
The Hydrogenation of Cyclopentadiene dimer
 |
 |
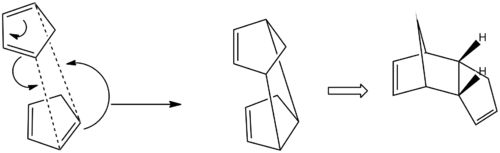
Cyclopentadiene can dimerise into an exo dimer or into an endo dimer however it dimerises specifically into the less stable endo dimer rather than the thermodynamically preferred exo product. Calculation of relative energies by MM2 algorithm from ChemBio3D Ultra 12.0 of the two dimers reveals a relative energy of 31.9 kcal/mol for the exo dimer but 34.0 kcal/mol for the endo dimer.
 |
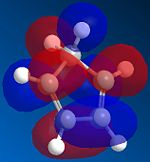 |
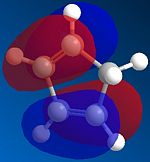
The reason is the Stereo-electronic or in other words the Kinetic control of the reaction. The alignment of cyclopentadienes in the fashion shown provides the best orbital overlap of the HOMO and LUMO, one from each cyclopentadine, for the reaction. However for the exo dimer to form the orbital overlap is destructive and does not allow the reaction to proceed.
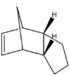
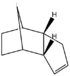
During the hydrogenation of cyclopentadiene both of the double bonds can be saturated although it is a lengthy process, where one double bond is reduced significantly faster. MM2 revealed the relative stability of each hydrogenated species demonstrating superior stability of product 2, which would predominate under thermal conditions. Kinetically controlled reaction should benefit the less hindered double bond, resulting in product 1, however the difference in steric hindrance between the two double bonds is infinitely small and the effects of kinetics on the reaction product can be considered irrelevant yielding the most thermodynamically stable product.
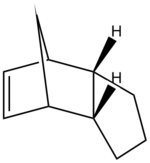 |
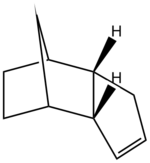 |
Stereochemistry of Nucleophilic additions to a pyridinium ring

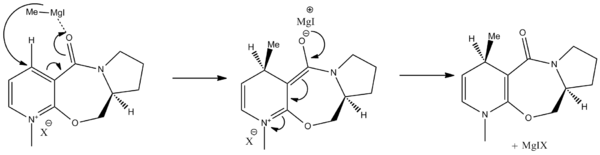
The prolinol derivative ring system 5 can be methylated by methyl magnesium iodide in the 4-position of the pyridine ring resulting in R-stereochemistry at the newly formed chiral centre. MM2 optimisation of geometry displays a torsion angle between the pyridine ring and the carbonyl of 12°, which means that the methyl is added from the more hindered face of the ring. Evidence suggests that the reason for the addition of methyl to the more hindered face of the ring is due to chelation effect of Magnesium in Grignard reagents to the carbonyl directing the nucleophilic attack from the top face, which does not happen with organolithium reagents resulting in random stereochemistry. DOI:10.1021/jo00356a016
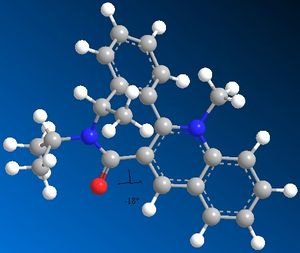

Based on earlier discussion the pyridinium ring 7 should react with Grignard reagents to add alkyl to the same face as the carbonyl group due to chelation effect however when reacted with aniline it adds to the opposite face as there is no positively charged metal cation to chelate with the carbonyl oxygen and steric control dominates the addition. MM2 demonstrates a -18° dihedral angle between the pyridine ring and the carbonyl thus the aniline is added to the top face of the molecule, which is further stabilized by dipolar interactions with the methyl of acetyl moiety DOI:10.1016/j.tetasy.2004.11.004 .
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
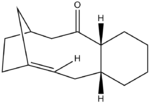 |
 |
Intermediate 9 or 10 is prepared in the total synthesis of Taxol, where these intermediates are atropisomers of each other. Atropisomerism arises due to increased rotational barrier around a single bond preventing free interconversion between two conformations, carbonyl group pointing up or down. On standing the intermediate prepared isomerises into the alternative form indicating that the less stable isomer is synthesized probably due to kinetic or electronic factors. MM2 and MMFF94 algorithms both demonstrate greater stability of intermediate 10 thus it is intermediate 9 that is prepared and subsequently isomerises into the more stable intermediate 10.
Furthermore to the atropisomerism demonstrated by intermediates 9 and 10 they both show unusual stability towards hydrogenation. They are so called hyperstable akenes DOI:10.1016/S0040-4020(97)00595-4 . Hyperstable alkenes are less reactive than other alkenes due to stabilizing interactions of vicinal and transannular hydrogens, which has to be disrupted in order for the reaction to proceed increasing the activation barrier for the process. However it should still be noted that the alkane form is thermodynamically preferred but the kinetical barrier is increased thus more difficult to overcome.
Introduction to Modelling Using semi-empirical Molecular Orbital Theory
Unlike the MM semi-empirical Molecular Orbital Theory approximates the valence electron wavefunctions increasing the applicability of modelling to reactions governed by electronic interactions or predicting the vibrational and NMR spectra. It can be considered as expansion to MM, which predefines the molecular geometry before molecular orbital calculations.
Regioselective Addition of Dichlorocarbene

Steric factors cannot distinguish between the two double bonds of molecule 12. One can argue that the chlorine atom is much larger than hydrogen hindering one side significantly more however the bottom face of the molecule is clearly exposed to electrophilic attacks with no evident distinction of either double bond thus this approach fails to explain observed regioselective addition.
MP6 a method employed by ChemBio3D Ultra 12.0 for approximation molecular orbitals revealed that the HOMO (Highest occupied molecular orbital) is predominantly located on the double bond below the chlorine atom. Subsequently the greatest and available electron density is located on the same double bond directing all electrophilic attacks towards this double bond.
Electrophilic addition of dichlorocarbene indeed adds regioselectively to this double bond as predicted DOI:10.1039/P29920000447 demonstrating the power of the method for predicting reactivity governed by elctronic interactions.
 |
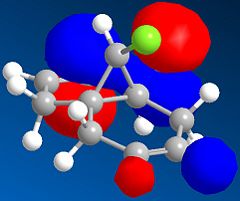 |
 |
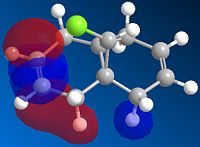 |
The double bond vibrations in predicted spectrum for molecule 12, 1737 and 1757 cm-1, have very low intensity compared to low energy bends of C-H bonds, high energy stretches of many C-H bonds spread over the entire molecule and C-Cl vibration, 770cm-1. The low intensity can be accounted for by the fact that there are many more C-H bonds than C=C, which greatly increases the intensity of the vibration.

Not surprisingly, when one of the double bonds is replaced with a single bond, one of the vibrations of C=C does not appear in the vibrational spectrum, 1753cm-1, confirming the absence of the bond and the nature of the species compared to molecule 12. Furthermore the intensity of C-H stretches increases as there are now even more C-H bonds that can be excited. Finally the nature of C-Cl vibration remains unchanged at 790cm-1 as the nature of the bond is not altered by replacement of the opposite double bond, which can be accounted in terms of the molecular orbitals calculated earlier. The molecular orbitals associated with C-Cl bond only extend to the endo double bond but not to the exo one as shown above.

Miniproject β-Lactam synthesis by Kinugasa cycloaddition [1] [2]
Intoduction

Stereocontrolled synthesis of β-Lactams became very important due to its presence in many antibiotics such as penicillins, β-Lactam acts as an inhibitor in bacteria cell wall construction, but also founds its application in anticancer agents or as a synthetic building block for complex molecules.
One of possible synthetic methods involve the Kinugasa reaction, a cycloaddition catalysed by Cu(I), which can results in the formation of two stereoisomers. However it was reported that the reaction proceeds in stereoselective fashion when N bears large substituents. In the reaction of Nitrone 1 with Acetylene 2 only the cis isomer forms in detectable quantity.

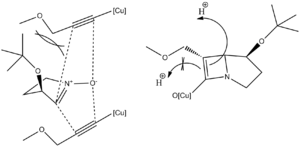
The argument proposed is a steric protection of one face by the substituent resulting in strereoselective addition to the opposite side of the molecule only.
Product was identified by 1HNMR (500 MHz, CDCl3) δ: 4.28 (1H, dt, J 6.9, 3.4 Hz), 3.70-3.62 (4H, m, H-3, H-5, H-6, CHHO), 3.56 (1H, dd, J 10.4, 8.0 Hz, CHHO), 3.38 (3H, s, Me) 2.96 (1H, dt, J 11.5, 7.6 Hz, H-30), 2.14 (1H, dtd, J 13.2, 8.2, 7.1 Hz, H-2), 1.92 (1H, ddt, 13.2, 7.5, 3.9 Hz, H-20), 1.21 (9H, s, t-Bu) and 13CNMR(125 MHz, CDCl3) δ: 177.5, 74.1, 70.0, 67.5, 62.7, 58.9, 51.4, 44.6, 39.2, 28.3, IR (film) 1765 cm-1¹
It should be noted that only 10 carbon shifts are observed as the tBu group rotates resulting in an average peak for all three carbons.
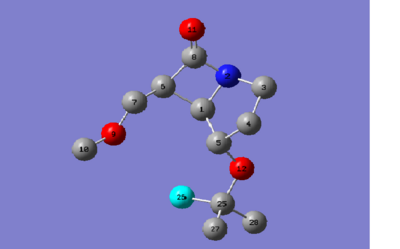
Calculated structure based on CNMR[3], IR and Hydrogen J couplings
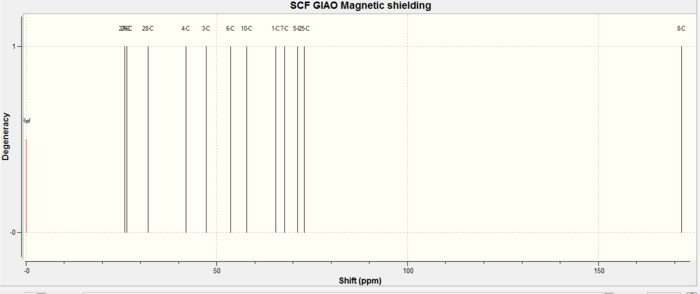
13CNMR (mPW1PW91/6-31G(d,p) GIAO) δ: 171.8, 72.9, 71.2, 67.8, 65.5, 57.8, 53.7, 47.2, 42.0, 32.0, 26.5, 25.9. The calculated CNMR presented here is for one possible conformation of the t-Bu group resulting in 3 chemical shifts one for each carbon, three lowest values, whereas measured spectra would result in average of 28.1, which is in good agreement with reported CNMR dif. 0.2ppm. Further adjustments need to be made to the amide carbon, highest chemical shift, based on previous findings 0.96δ + 12.2 = 177.1ppm dif. of 0.4ppm. Remaining C shifts differ by 2.8-0.3 with an average of 1.9ppm. In conclusion the calculated spectra supports the proposed carbon framework however by itself it cannot justify the stereochemistry assigned.
|
|
The hydrogen on C5 on picture above is predicted to show a dt J 8.5 Hz as calculated by Janocchio, the other coupling constant is more difficult to predict but is not necessary. There are two reported H dt where neither has a coupling constant J 8.5 Hz. Similarly with the trans isomer the predicted J 5.1 Hz does not match any reported value and it should be assumed that the predicted J values are inaccurate as they are only based on Karplus equation.
|
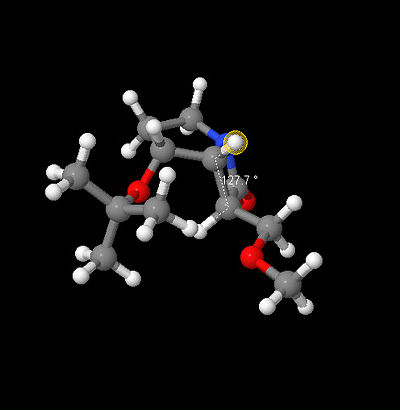
|
The only reported IR stretch is at 1765 cm-1, which presumably corresponds to the carbonyl and was confirmed by b3lyp/6-31G(d,p) calculation, however the resulting value is 1861 cm-1, a reasonable agreement.
Conclusion
In conclusion the calculated carbon framework and functionality are in a good agreement with reported values supporting the structure proposed. However the absolute stereochemistry of the molecule cannot be confirmed nor discredited as clearly the calculated J values fail to support either structure at the stereocentre in question. In the future better calculation of J couplings would be necessary to support the structure proposed, whereas for now the mechanistic approximation of stereochemistry remains the only prediction possible.
Literature
- ↑ A. Mames, S. Stecko, P. Mikozajczyk, M. Soluch, B. Furman, M. Chmielewski, J. Org. Chem, 2010 DOI:10.1021/jo101355h
- ↑ A. Mames, S. Stecko, B. Furman, M. Chmielewski, J. Org. Chem, 2008, 73, 7402-7404 DOI:10.1021/jo801212q
- ↑ Calculated CNMR: DOI:10042/to-5254