Rep:Module3:Dima1407
Optimizing 1,5-hexadiene
| Conformer | Structure | Point Group | Energy/Hartrees | Relative Energy/kJ/mol | ||
|---|---|---|---|---|---|---|
| Gauche1 | C2 | -231.68772 | 12.97 | |||
| Gauche2 | C2 | -231.69167 | 2.60 | |||
| Gauche3 | C1 | -231.69266 | 0.0 | |||
| Gauche4 | C1 | -231.69153 | 2.97 | |||
| Anti1 | C2 | -231.69260 | 0.16 | |||
| Anti2 | Ci | -231.69254 | 0.31 | |||
| Anti3 | C2h | -231.68907 | 9.43 | |||
| Anti4 | C1 | -231.69097 | 4.44 |
We have obtained the same results optimizing our structures as in the table in Appendix1.
The energy obtained in optimization at the higher level of theory is -234.55972 Hartrees. The energy difference is very big. But geometry obtained in both cases are very similar - maximum angle difference is 1 degree between carbon atoms and less than 0.2 degrees between Hydrogen-Carbon-Hydrogen atoms.
Angles at high theory level:
C1-C2-C3 =125,23
C2-C3-C4 = 112.65
Angles at low theory level:
C1-C2-C3 =124.81
C2-C3-C4 = 111.35
We have done a vibrational analysis and have not found any imaginary (negative) frequencies - the structure is energy minimum.
The information obtained from Thermochemistry part:
Sum of electronic and zero-point Energies= -234.416239
Sum of electronic and thermal Energies= -234.408960
Sum of electronic and thermal Enthalpies= -234.408016
Sum of electronic and thermal Free Energies= -234.447788
Optimizing the "Chair" and "Boat" Transition Structures
From the guess transition state optimization we can see that chair TS is lover in energy than boat TS.
Chair TS = -231.61932 Hartree
Boat TS = -231.60280 Hartree
Energy difference = 0.01652 Hartree (or 43.4kJ/mol)
Boat TS has an imaginary frequency of magnitude 839 cm-1and Chair TS has an imaginary frequency of magnitude 818 cm-1
Using the frozen coordinate method the result is differed in seventh decimal place, so those methods are similar.
QST2 method is good when you can not predict the TS, but it also has disadvantages - for example it could not be used for predicting chair TS, only Boat one. The predicted Boat TS energy is the same as in guess transition state optimization. E = -231.60280 Hartree.
IRS predicts us Gauche2 isomer formation from Chair TS DOI:10042/to-6305 and Gauche3 isomer formation from Boat TS DOI:10042/to-6345 .
| Electronic energy | Sum of electronic and zero-point energies (Hartree) | Sum of electronic and thermal energies (Hartree) | ΔE at 0 K (kcal/mol) | ΔE at 298.15 K (kcal/mol) | Expt.at 0 K (kcal/mol) | |
|---|---|---|---|---|---|---|
| Chair TS | -234.505467 | -234.362663 | -234.356753 | 33.62 | 32.76 | 33.5 ± 0.5 |
| Boat TS | -234.492915 | -234.351359 | -234.345054 | 40.71 | 40.10 | 44.7 ± 2.0 |
| Reactant (Anti2) | -234.559715 | -234.416239 | -234.408960 |
The Diels Alder Cycloaddition
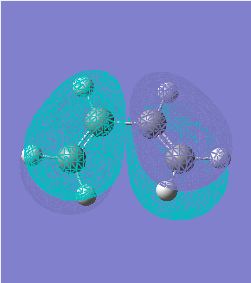
HOMO(-0.344) and LUMO(0.017) orbitals of butadiene:
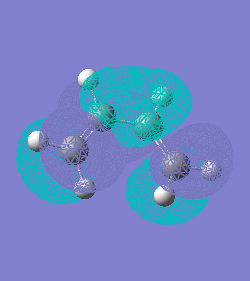
HOMO(-0.387) and LUMO(0.052) orbitals of ethylene:
Butadiene's HOMO is anti-symmetric, LUMO is symmetric.
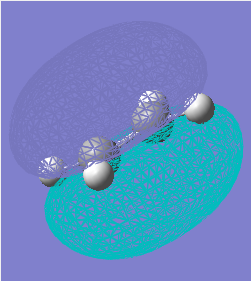
The transition structure maximizes the overlap between the ethylene π orbitals and the π system of butadiene:
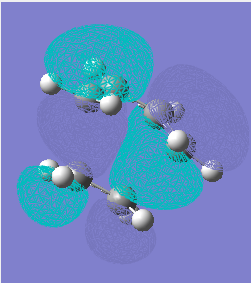
TS HOMO and LUMO:
The TS HOMO is anti-symmetric, LUMO - symmetric. HOMO is formed from The bond length of the partly formed σ C-C bond is 0.212nm, while typical sp3 and sp2 C-C bond lengths are 0.154nm and 0.148nm[1]. The Carbon van der Waals radius is 0.17nm[2]. So the TS distance is smmaller than 2 van der Waals radius and bigger than proper C-C bond.
The vibration that corresponds to the reaction path at the transition state is imagine with the magnitude of 956cm-1 The formation of the two bonds is synchronous.