Rep:Module2rh1909
Module 2
Structural Analysis of TlBr3
The ground state geometry of TlBr3 was optimised using the 'B3LYP' method. The medium level basis set of LanL2DZ was used, which is suitable for heavier elements like those in question here.
The optimisation gave the following results:
|
File Type |
.log |
|
Calculation Type |
FOPT |
|
Calculation Method |
RB3LYP |
|
Basis Set |
LanL2DZ |
|
Charge |
0 |
|
Spin |
Singlet |
|
E(RB3LYP) (a.u.) |
-91.22 |
|
RMS Gradient Norm (a.u.) |
0.00 |
|
Dipole Moment (Debye) |
0.00 |
|
Point Group |
D3H |
|
Tl-Br Bond Distance (Angstroms) |
2.65 |
|
Br-Tl-Br Bond Angle (o) |
120.0 |
You can find the TlBr3 optimisation file by clicking this link. Media:TLBR3_OPTIMISATION.LOG
The Tl-Br bond distance is in relatively good agreement with the observed value of 2.52 Angstroms.[1] The bond angle is also as expected for this molecule with D3H point group symmetry.
The optimised TlBr3 molecule was then subjected to a frequency analysis. It is vital that the same method and basis were used otherwise the results are not relative and are uncomparable. The optimised structure is found by the computer after a series of trial and error calculations until the gradient of the Potential Energy Surface curve is equal to zero. The frequency analysis must be carried out in order to confirm that the optimised structure is at the energy minimum and not a maximum. This is done by calculating the second derivative of the PES curve. For the structure to be at an energy minimum, none of the frequency values should be negative. This was the result obtained, all frequencies and intensities are listed in the table below:
|
Vibrational Mode No. |
Frequency (cm-1) |
Intensity |
|
1 |
46 |
4 |
|
2 |
46 |
4 |
|
3 |
52 |
6 |
|
4 |
165 |
0 |
|
5 |
210 |
25 |
|
6 |
210 |
25 |
See here for a link to the frequency analysis file. Media:ROSEANNA_TLBR3_FREQACTUAL.LOG
This file tells us that the 'low frequencies' are -3, 0, 0, 0, 4 and 4cm-1. These frequencies correspond to vibrations of the centre of mass of the molecule and are much smaller than the vibrational modes seen in the table. The table shows that the lowest “real†normal mode is 46cm-1, this corresponds to two vibrational different vibrational modes and would be found at the energy minimum. The first vibration involves two of the bromine atoms moving towards and away from each other with other atoms stationary. The second vibration involves only one bromine atom moving from side to side with all other atoms stationary.
The IR spectrum shows only two peaks. This is because one of the vibrational modes would not show up as it is completely symmetric (all bromine atoms moving in and out in concerted manner with thallium stationary). The other four vibrations are paired off into two sets of degenerate vibrations, so there is no separation of peaks.
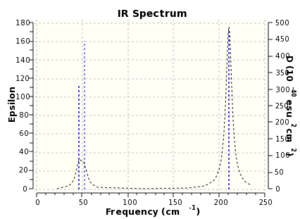
The spectrum is in good agreement with observed literature values for vibrations.
Molecular Orbital Analysis of BH3
Using the ‘B3LYP’ method with a basis set of ‘3-21G’ a
molecule of BH3 was optimised. The resultant molecule was viewed in Gaussview and it appeared that the bonds had disappeared.
Of course, they haven’t disappeared in reality, it’s
just that the optimised value for the bond distance is outside a value that was
previously defined by Gaussian.
In reality, a bond is an overlapping of orbitals between two
atoms, and the electron density from both atoms is spread across the new
molecular orbital (of course depending on other properties such as
electronegativity). One model describes the LCAO method, whereby atomic
orbitals of the same symmetry are linearly combined to give molecular orbitals.
These can be depicted in an MO diagram, which has been drawn in this section,
along with qualitatively calculating the MOs using Gaussian.
The optimised BH3 molecule was then subjected to
a frequency analysis in order to confirm the structure was at an energy
minimum. The table below describes the form of the vibrations, their frequency
and intensity, and the symmetry of the vibration in the D3H point
group.
|
Form of Vibration
|
Frequency (cm-1)
|
Intensity
|
Symmetry D3H Point Group
|
|
1 All atoms moving up and down. Three Hs moving in opposite direction to B. ADD MOVIE
|
1144
|
93
|
a2”
|
|
2 Two Hs moving simultaneously towards each other, with other B-H stationary
|
1204
|
12
|
e’
|
|
3 Two Hs rock left to right in seesaw motion, whilst other H moves left and right simultaneously
|
1204
|
12
|
e’
|
|
4 All Hs move in and out in a concerted manner with B stationary
|
2598
|
0
|
a1’ totally symmetric
|
|
5 Two Hs move in and out in unconcerted manner with other H stationary
|
2737
|
104
|
e’
|
|
6 Two Hs move in and out simultaneously, and other H moves in out in unconcerted manner
|
2737
|
104
|
e’
|
As you can see, there are no negative frequencies, so the optimised structure is at an energy minimum. Media:ROSEANNA_BH3_FREQ.LOG. The IR spectrum reflects the data. There are only three peaks shown as the dipole moment in vibration 4 is cancelled out due to total symmetry, and there are two
sets of degenerate vibrations.
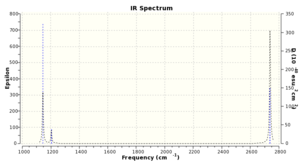
.
The MOs of the optimised structure were then investigated,
and the population analysis can be found by clicking this link. DOI:10042/to-12894 .
Please find here, a diagram of a drawn MO diagram of BH3
depicting the LCAOs and the MOs calculated from the population analysis.
Media:BH3 Molecular Orbital Diagram.docx.
The qualitatively calculated MOs are very similar to the
LCAOs for the bonding (and non-bonding) orbitals. So for calculation of the
occupied orbitals, the qualitative modelling method seems very accurate. For
the anti-bonding orbitals, however, the qualitative MOs have quite different,
odd-shaped MOs. For example, the MO corresponding to
the 3a1’ anti-bonding LCAO, is very different. It
actually appears that this MO and the MO for one of the higher energy
anti-bonding orbitals should in fact be switched round! This would suggest that the quantum
mechanical calculations for determining the energies for
these orbitals was not very accurate. In practise however, the need to
know a lot of information about these orbitals may not be necessary, because
they are so high in energy compared to the HOMO that they may never be occupied
or involved in the chemistry of the molecule.
Analysis of the cis and trans Isomer of Mo(CO)4(PCl3)2
The purpose of this section is to analyse the two isomers to
find out which one is more stable. Each isomer was first optimised using the
‘LanL2MB’ basis set, the torsion angles between Cl-P-Mo-C
were then altered appropriately in the resulting molecules. They were then
subjected to another optimisation using the ‘LanL2DZ’ basis set. For the
optimisation files for the cis isomer, click this link here. DOI:10042/to-12895 . For the trans isomer, click this link
here. DOI:10042/to-12897 .
These isomers can thermally interchange between one another.
The results show that the total energy for the cis-isomer is -1637211kJ/mol and for the trans-isomer
is -1637208kJ/mol. This means that the trans-isomer
is less stable by 3kJ/mol. The calculations for energy are accurate to around
10kJ/mol,[2] so these results cannot be verified.
It would be expected that the trans-isomer
would be the most stable, mainly due to steric reasons.[3]
By changing the R groups on the PR3 ligand, you
can ‘fine-tune’ the complex in order to make one or the other isomer more
stable. Increasing the steric bulk of the PR3 ligand,
greatly increases the stability of the trans-isomer,
e.g. PPh3.[4] In order to make the cis-isomer, a bidentate
phosphine ligand could be used.
The frequency analysis of the cis-isomer can be found here. DOI:10042/to-12899 . In the CO stretching region, there are four peaks.
|
Frequency (cm-1)
|
Form of vibration
|
|
1945
|
Equatorial COs move in and out in unconcerted manner
|
|
1949
|
Axial COs move in and out, unconcerted
|
|
1958
|
Axial COs move in and out in a concerted manner, whilst equatorial COs move OUT and in
|
|
2023
|
All COs move in and out in concerted fashion
|
There are some very low frequencies present in the results at 11cm-1 and 18cm-1. After animating the vibration, it was seen that they correspond to a twist in the Mo-P bond. For example, the vibration corresponding to 11cm-1, involves Mo-P bond twisting in opposite directions causing the whole complex to tilt forwards and backwards in
an oscillating motion.
These vibrations are of such low energy that they will
be occurring rapidly and constantly at room temperature.
The frequency analysis of the trans-isomer can be found here. DOI:10042/to-12900 . This
time in the CO stretching region there is only one peak.
|
Frequency (cm-1)
|
Form of vibration
|
|
1950.47
|
Two equatorial trans-COs move in and out in concerted manner
|
|
1951.12
|
Other two equatorial trans-COs move in and out in concerted manner
|
There is only one peak for the trans-isomer compared to the cis-isomer due to the increase in symmetry of the CO groups in the trans-isomer. If you look at the frequencies in the CO stretching region, there are actually four, however, these correspond to totally symmetric vibrations, therefore no dipole moment, and no intensity on the IR spectrum. The cis-isomer has COs in non-symmetric positions, therefore the CO stretched are all visible on the spectrum.
There are also some very low frequencies present in the
results for the trans-isomer, e.g.
5cm-1 and 6cm-1. These also correspond to a twisting of
the P-Mo bond. For example, the vibration corresponding to 5cm-1 is
a twisting of P-Mo bonds in same direction. The fact that these vibrations are
of such a low energy, suggests that the P-Mo bond itself must be relatively
weak. This is as expected because the PCl3 ligand is known to be a
poor π-donor
(due to electronegative chlorine atoms).[5] This
also helps to explain the high frequency values for both complexes. As PCl3
is donating little to the metal centre, less electron density is available for
Mo-C back-donation, and therefore the CO bond remains very strong.
Below are bond distances and angles measured for each isomer.
|
Bond or Angle
|
Cis-isomer (distance in angstroms, angle in deg)
|
Trans-isomer (distance in angstroms, angle in deg)
|
Difference
|
|
Mo-P
|
2.51
|
2.44
|
0.07
|
|
Mo-C(eq)
|
2.01
|
2.06
|
-0.05
|
|
Mo-C(ax)
|
2.06
|
-
|
-
|
|
C-O
|
1.17
|
1.17
|
0
|
|
P-Cl
|
2.24
|
2.24
|
0
|
|
Mo-P-Cl
|
116.0
|
117.3
|
-1.3
|
|
Cl-P-Cl
|
100.2
|
99.1
|
1.1
|
The Mo-P bond in the cis isomer is 0.07 angstroms longer than in the trans isomer. This is as expected if the trans isomer is more stable. The steric strain in the cis isomer weakens the Mo-P bond slightly. The Mo-P-Cl bond angle is 1.3o smaller in the cis isomer. The PCl3 ligand is under more strain in the cis isomer, this is demonstrated by this result. All bond distances and angles are comparable to literature values.[6]
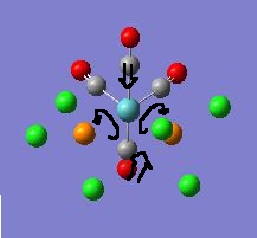
Exploring Bonding in Aluminium Halides
It is known that aluminium halides Al2X6
(X = Cl, Br) form 3 centre-2 electron bonds, with the
halides in a bridging position. In this modelling experiment, the bonding
situation will be investigated for three isomers of Al2Br2Cl4
in order to determine the effect of bromine atoms on the compound and the
stability of each isomer. The three isomers in question are shown in the
picture.
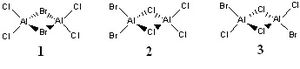
First, the bonding in Al2Cl6 will be
investigated. Before being optimised, the bond lengths and angles were altered to
be as near to observed values as possible. [7]. The
molecule was then subjected to an optimisation and then frequency analysis
using the ‘B3LYP’ method with the ‘6-31G’ basis set. The total energy of this
molecule was found to equal -3246.320a.u. Click these links for the result of
these analyses. Optimisation file DOI:10042/to-13028 . Frequency analysis DOI:10042/to-13030 .
Below is a table describing the geometry of the compound. As expected for a 3c-2e bond, the bridging
chlorine atoms have longer bond lengths than the terminal ones.
|
Bond Distances (Angstroms)
|
Bond Angles (o)
| ||
|
Al-Clt
|
2.17
|
Clt-Al-Clb
|
110.3
|
|
Al-Clb
|
2.41
|
Clb-Al-Clb
|
86.4
|
|
Clt-Al-Clt
|
123.3
|
Below is a table showing the results of the frequency analysis. The IR spectrum shows seven peaks. The higher frequency vibrations correspond to movements of the aluminium atoms, and the lower frequencies correspond to chlorine atom vibrations. This is expected because the chlorine atoms are more freely able to move without affecting the whole molecule (compared to the aluminium atoms).
|
No.
|
Form of Vibration
|
Frequency (cm-1)
|
Intensity
|
|
1
|
Al atoms move up and down, concerted, with all other atoms stationary
|
580
|
258
|
|
2
|
Al atoms move side to side, concerted, with all others stationary
|
437
|
365
|
|
3
|
Al atoms move into and out-of-plane
|
355
|
153
|
|
4
|
Clb atoms shorten and lengthen in concerted manner
|
277
|
103
|
|
5
|
Clb atoms move up and down in concerted manner
|
159
|
18
|
|
6
|
Clt atoms move towards and away from each other, causing seesaw motion of molecule
|
124
|
25
|
|
7
|
Clt atoms move into and out-of-plane, concerted.
|
113
|
14
|
In order to analyse the bonding correctly, (and to calculate the energy of formation of the dimer), two AlCl3 fragments will be used, and the NBO analysis results studied. The optimisation and frequency analysis of this fragment were carried out using the same method and basis set as for the dimer. Optimisation file: DOI:10042/to-13033 . Frequency analysisDOI:10042/to-13034 . The total energy of the fragment was found to be equal to -1623.144a.u. This can be used to calculate the energy of formation via this equation:
ΔE = E(Al2Cl6)-E(AlCl3)+E(AlCl3)
This gives a value of -83.49kJ/mol
(4s.f.)
The .log file containing the NBO analysis was then studied: DOI:10042/to-13035 . This describes the bonding in the molecule,
and we are able to see that an sp2
hybridised Al orbital forms a bond with a chlorine atomic p-orbital. Looking at
the ‘Perturbation Theory Analysis’ section, gives us details of the acceptor
and donor ability of each atom in the molecule and can be used to see why AlCl3
readily dimerises. These results shows that if a lone
pair on a chlorine atom (LP) were to donate itself into an empty p-orbital on
an aluminium atom (LP*) this gives a stabilisation energy (E(2))
of 67.82kJ/mol. This gives the largest stabilisation energy of all possible
donations, and so would be the expected method of bonding in the dimer.
Now to look at the three Al2Br2Cl4
isomers. Below is a table showing the geometries of the three isomers.
See optimisation files here. Isomer 1: DOI:10042/to-13036 . Isomer 2: DOI:10042/to-13037 . Isomer 3: DOI:10042/to-13038 .
|
Bond Distances (Angstroms)
|
Bond Angles (o)
| ||||||
|
Isomer 1
|
Isomer 2
|
Isomer 3
|
Isomer 1
|
Isomer 2
|
Isomer 3
| ||
|
Al-Clt
|
2.18
|
2.17
|
2.17
|
Clt-Al-Clt
|
123.0
|
122.5
|
-
|
|
Al-Brt
|
-
|
2.29
|
2.29
|
Clt-Al-Brt
|
-
|
-
|
124.3
|
|
Al-Clb
|
-
|
2.42
|
2.41
|
Brt-Al-Brt
|
-
|
129.1
|
-
|
|
Al-Brb
|
2.52
|
-
|
-
|
Clt-Al-Clb
|
-
|
110.3
|
110.3
|
|
Clt-Al-Brb
|
109.6
|
-
|
-
| ||||
|
Brt-Al-Clb
|
-
|
108.2
|
108.2
| ||||
|
Clb-Al-Clb
|
-
|
86.7
|
87.2
| ||||
|
Brb-Al-Brb
|
90.4
|
-
|
-
|
These results show that the bromine bridging bond is larger than the chlorine bridging bond and possible, therefore, less stable. This could be due to steric strain in the four atom central ring.
Below is a table showing the results of the frequency
analysis for all three isomers. The frequencies have been paired up with the
same vibrations described for Al2Cl6. See frequency
analysis files here. Isomer 1: DOI:10042/to-13039 . Isomer 2: DOI:10042/to-13040 . Isomer 3: DOI:10042/to-13041 .
|
Vibration No.
|
Frequency (cm-1)
|
Intensity
| ||||
|
Isomer 1
|
Isomer 2
|
Isomer 3
|
Isomer 1
|
Isomer 2
|
Isomer 3
| |
|
1
|
571
|
571
|
561
|
239
|
160
|
249
|
|
1(a)
|
-
|
531
|
-
|
-
|
88
|
-
|
|
2
|
431
|
465
|
399
|
321
|
91
|
396
|
|
2(a)
|
-
|
388
|
-
|
-
|
293
|
-
|
|
3
|
332
|
353
|
352
|
144
|
137
|
137
|
|
4
|
233
|
289
|
260
|
93
|
38
|
54
|
|
4(a)
|
-
|
250
|
-
|
-
|
27
|
-
|
|
5
|
126
|
152
|
149
|
11
|
15
|
14
|
|
6
|
112
|
109
|
109
|
16
|
16
|
19
|
|
7
|
103
|
108
|
107
|
11
|
9
|
10
|
From the IR spectra, you can see that isomer 2 has three more peaks than 1 & 3. This is because it is less symmetrical compared to the other isomers. These extra peaks correspond to vibrations 1(a), 2(a) and 4(a). The pairs of vibrations correspond to one large stretching motion on the terminal chlorine side and little on the bromine side, and vice versa for the other vibration (a) in the pair. As a result, the intensities of these vibrations are also smaller than for the other isomers. The majority of the frequencies for isomer 2 are all also higher energy than the others. This may be due to the two terminal bromine atoms being in a closer proximity to each other.
Using the same method as for Al2Cl6,
the energy of formation of the three isomers was calculated.
|
Isomer 1
|
Isomer 2
|
Isomer 3
| |
|
Total Energy (a.u.)
|
-7469.1311
|
-7469.1311
|
-7469.1312
|
|
ΔE (kJ/mol)
|
-104
|
-105
|
-106
|
As you can see, there is little difference between the total energies of the molecules and the energy of formations. However, one thing to notice is the difference between these values for ΔE and the value of ΔE for Al2Cl6, which is considerably ‘less negative’, showing that the addition of bromine into these molecules makes them overall more stable.
In order to calculate which isomer is most stable, we must look at the NBO analysis of each possible
fragment. These fragments are AlBrCl2 and AlBr2Cl. File for AlBrCl2 fragment: DOI:10042/to-13042 . File for AlBr2Cl fragment: DOI:10042/to-13043 . For
isomer 1, the .log file was studied and showed that the stabilisation energy, E(2), for the dimerization where a lone pair in a p-orbital
on a bromine atom (LP) was to be donated into the antibonding
Al-Cl bond (BD*) is 29.00kJ/mol. All results are
tabulated below.
|
Orbitals involved in Donation
|
Stabilisation Energy (kJ/mol)
| |
|
Isomer 1
|
p-Br LP à Al-Cl BD*
|
29.00
|
|
Isomer 2
|
p-Cl LP à p-Al LP*
|
69.71
|
|
Isomer 3
|
p-Cl LP à Al-Br BD*
|
66.74
|
From these results, you can see that the most stable isomer is isomer 2. The stabilisation energy for isomer 1 is more than half the other two, showing bromine’s in a bridging position are less stable than chlorine atoms.
Isomer 2 shows different orbitals being involved in the bond
formation. Rather than the chlorine lone pair being
donated into the Al-X antibond, it is donated into an
empty non-bonding p-orbital of lower energy.
Conclusion
As you can see, the in-depth study of Al2Cl6
and the three isomers of Al2Cl6 showed a number of
things. The optimisation and frequency analysis of Al2Cl6
showed that it’s energy of formation is ‘less
negative’ than when bromine atoms are involved, showing that bromine stabilises
the isomers in some way.
The geometric analysis of all compounds showed that the
bridging bonds were longer than the terminal ones. This is in accordance with
the known 3c-2e bonding present in these molecules. The bridging bonds are
weaker and have a lower bond order because of this.
The frequency analysis of all compounds showed that the
higher energy vibrations are due to movement of aluminium atoms, with
vibrations largely involving the halide atoms at lower energy vibrations. There
appears to be little difference between chlorine and bromine vibrations.
The total energies and ΔE values are very similar
for all three isomers. The NBO analysis of all three isomers allowed us to find
out the stabilisation energy upon donation of electrons to the aluminium atoms.
These results were more decisive and showed that isomer 2 was the most stable.
- ↑ J. Blixt et al., J. Am. Chem. Soc.,1995, 117(18), 5089: DOI:10.1021/ja00123a011
- ↑ http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year3/8a_accuracy.html
- ↑ D.J. Darensbourg & R.L. Kump, Inorg. Chem.,1978, 17(9), 2680: DOI:10.1021/ic50187a062
- ↑ T. Kinnunen et al., J. Organomet. Chem., 2003, 665, 150: DOI:10.1016/S0022-328X(02)02107-1
- ↑ E.C. Alyea et al., Inorg. Chem.,1995, 34, 3864: DOI:10.1021/ic00119a006
- ↑ F.A. Cotton et al., Inorg. Chem.,1982, 21(1), 295: DOI:10.1021/ic00131a055
- ↑ K. Aarset et al., J. Phys. Chem. A,1999, 103(11), 1644-52: DOI:10.1021/jp9842042
