Rep:Module2:inorganicwm207
This module aims to subject inorganic chemistry to computational methods to see what we can learn.
Small Molecules
BCl3
BCl3 is a small inorganic molecule which can be modelled reasonably accurately by computational methods. The main difficulty lies in the Chlorine atoms because they are in the second row of the periodic table and contain many non-bonding shells of electrons. The solution to this is to use a "LANL2MB" calculation method which treats the valence elctrons as normal but also estimates the effects of the non-valence electrons. However, this is not the most refined calculation method; the "MB" stands for minimal basis set. The total term ("LANL2MB") combines a medium level basis set "D95V" on first row atoms and pseudo-potentials on heavier elements.
It is necessary to use the same basis set for all calculations you wish to compare because they are each associated with different degrees of error and they work in different ways. These calculations are not exact, they are more comparable with pratical methods and as such results by different methods simply don't compare.
Once the structure has been optimised by Gaussian (that is to say all interactions have been considered and the lowest possible energy of the molecule has been found) it is necessary to double check via frequency analysis. Gaussian makes small changes to the molecule and calculates the effect on the total energy (moving in such a direction as to lower the energy at all times). It stops when it finds an energy minima, where the gradient of the change in energy goes to zero. However, this is also a property of maxima and frequency analysis serves to verify the optimisation has finished at a minima and not a maxima.
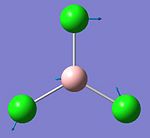
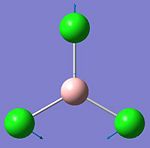
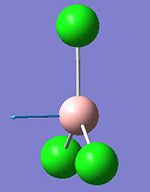
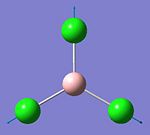
They vibrational modes of BCl3 are documented on the left. Once the calcultion has been run, the output file is opened in Gaussview which allows you to animate the vibrations. For simplification purposes the still pictures have been included here with vectors added to indicate the direction of the vibration. This information is useful in itself not purely because it can verify the location of the energy minima but also because it is these vibrational modes that are being excited when we subject physical samples to IR Spectroscopy. Because the calculation returns such quantitative values for frequency and intensity it is possible to plot these to form a computer-generated IR Spectrum, shown below.
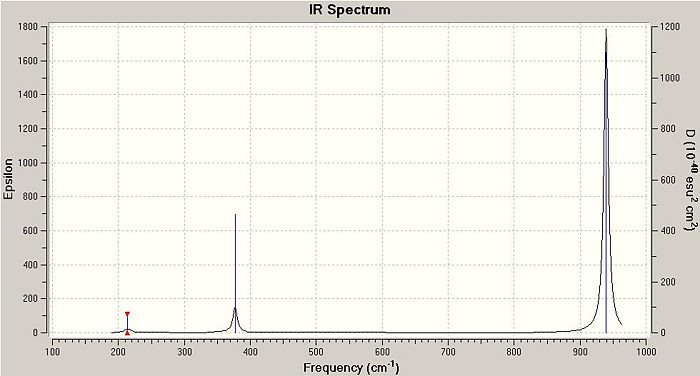
This calculation was submitted with D3h symmetry tightly bound. Gaussian defines the symmetry itself before commencing calculations as this saves a lot of time, although Gaussian only loosely enforces these symmetry elements. By submitting the job with D3h symmetry tightly bound it was possible to speed up the calculation as this is the expected ground state symmetry. Gaussian also defined the C2v and C2 axes and the job was run in approximately 8 seconds.
BH3
MO analysis
Initial Optimisation of BH3 was successful and calculation of its molecular obrbitals agreed with the MO diagram below. The only notable difference was the promotion of the 3a1' above the 2e' non-bonding orbitals. Although, this does not suggest any failing on the part of the calculation because LCAO theory can only guess at the strength of s-s interactions compared with s-p and the relative energy of a1' with respect to e' energy levels. In fact the computational approach can be praised here for giving a fully quantitative solution to the problem of which orbital should be placed higher. In contrast LCAO theory does not appear so successful, although it must be noted that the MO diagram was first produced by LCAO theory and it was right in all aspects but one which it couldn't properly predict. This is a small failing in a method which does not require the processing resources of computer aided calculations and they can still be used for a general understanding of molecular bonding.

|
The output checkpoint file to read the MO calculations is available here: https://www.ch.ic.ac.uk/wiki/index.php/Image:Wm207_bh3_pop.chk
Frequency Analysis
The Vibrational modes of BH3 were again calculated by a frequency analysis, initially to confirm the correct optimisation of the molecule. The 6 modes are displayed on the left and it is interesting to note the significant differences to the BCl3 example above are that it is the lighter element that is more easily displaced. Above, Boron was often displaced, moving around in a ring of much heavier Chlorine atoms which stayed fairly still. Whereas, in this example it is quite plainly the Hydrogens which are displaced to the greatest extent, much more so than the corresponding vibrations of Chlorine atoms where the vibration is not so exaggerated.

Cis/Trans-Isomer of Mo(CO)4L2
This exercise aims to make more use of the IR Spectra produced by Gaussview by using them to compare the cis/trans isomers of Mo(CO)4L2. This is in fact a revision of an inorganic synthesis experiment from the 2nd year labs where L was in fact PPh3. As phenyl groups are large and contain many atoms this slows calculations down to a large extent. Is is common, therefore, to replace phenyl groups with Chloring atoms which are regarded as having similar electronic behaviour in respect to their neighbouring atoms. The theory in determining between these compounds using IR Spectra is the differing symmetry of each molecule. The trans-isomer has 4 equivalent Carbonyl groups so when their IR Stretching is analysed only one peak should be found for Carbonyls as they will all be stretching at the same frequency. On the other hand, the cis-isomer has 2 environments for Carbonyls, trans to another carbonyl or trans to an L-group, this molecule is expected to produce 4 Carbonyl peaks on the IR Spectrum.
As before it is always necessary to start by optimising the molecule. This was first done by creating the molecule, with PCl3 for L, and subjecting it to optimisation by Gaussian with a LAN2MB basis set and additional keywords "opt=loose".
The product of this calculation was rather surprising in that Gaussian had removed all of the bonds in the PCl3 groups and their bonds to the metal centre. It has been previously discussed that Gaussview has distance related parameters to determine the visual presence of bonds, despite the fact it can see the electron density between the two atoms which indicates the presence of a bond it chooses not to draw one.
| cis-isomer | trans-isomer |
|---|---|
 |
|
cis-isomer DOI:10042/to-3485 / trans-isomer DOI:10042/to-3486
The bonds were redrawn so that the orientation of the PCl3 could be specified. For the cis-isomer one P-Cl bond was made to have a torsion angle of 0 degrees relative to a cis-carbonyl and a P-Cl bond on the other ligand was made to have a torsion angle of 180 degrees to the same cis-carbonyl. For the trans-isomer the PCl3 ligands were made to be eclipsed and that one P-Cl bond would be aligned with a carbonyl group.
The optimisation calculation resubmitted, this time with a higher level basis set of LAN2DZ and the additional keywords "int=ultrafine scf=conver=9".
Once again the calculation was returned without bonds between the P and Cl atoms.
| cis-isomer | trans-isomer |
|---|---|
 |
|
cis-isomer DOI:10042/to-3487 / trans-isomer DOI:10042/to-3488
Unfortuantely Gaussview is not able to request calculations concerning the empty d-orbitals of phosphorus although they play a large part in the electronic structure of phosphorus, especially as a ligand. However, it is possible to adulterate the *.gif (gaussian input file) to include these in the calculations. This involves opening the *.gif with a text editing application (such as Notepad) and adding "extrabasis" to the line which contains "int=ultrafine scf=conver=9". It is then necessary to append:
(blank line)
P 0
D 1 1.0
0.55 0.100D+01
(blank line)
to the end of the file. The *.gif can then be submitted to the SCAN for calculation and the resulting output file will have the benefit of the extra d-orbital interactions of phosphorus.
| cis-isomer torsion angle 0 degrees |
cis-isomer torsion angle 180 degrees |
trans-isomer eclipsed |
|---|---|---|
 |
 |

|
The output files are: DOI:10042/to-3489 cis-isomer / DOI:10042/to-3490 trans-isomer.
When the output file was determined to have completed successfully a frequency analysis was also submitted, with the same LAN2DZ basis set, keywords "int=ultrafine scf=conver=9 pop=(full,nbo)" and adulterated as before to include "extrabasis" and the code for the d-orbitals of Phosphorus.
The output files were unable to be published.