Rep:Module1rh1909
Computational Chemistry Lab - Module 1
Modelling Using Molecular Mechanics
Dimerisation of Cyclopentadiene
The dimerisation of cyclopentadiene could in theory yield two isomers, the exo or endo product.
Using the MM2 force-field on ChemBio3D, the geometries of the two potential isomers can be
optimised and the total energy of each compound can be found.
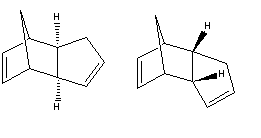
The total energy for the exo product is 31.8765kcal/mol and for the endo product is 33.9975kcal/mol. These results show that the exo product is the more stable isomer and is the product if the dimerisation was under thermodynamic control. In practise, the endo product is the major, and sometimes only, product formed showing that the dimerisation is under kinetic control.i This means that the transition state of the endo product must be relatively stable compared to the exo. Studies have shown that in Diels-Alder reactions, there is a considerable amount of overlap between HOMOs and LUMOs in the endo conformation which have strong orbital symmetry. Not only are there primary overlaps in the formation of the s-bonds, but there are secondary overlaps creating more stability in the transition state.ii These secondary interactions are not present in the exo transition state.
Hydrogenation of Cyclopentadiene Dimer
Hydrogenation of the endo product can potentially occur at either double bond. Once again using
the MM2 force-field, energy values for both products can be calculated.
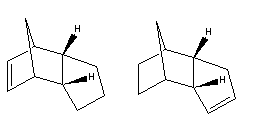
The total energy values show that 3 is the kinetic product and 4 is the thermodynamic product. By looking at the contributions from stretching, bending, Van der Waal's and H-bonding energies, you can see that for both compounds, the bending energy contributes 18.7811kcal/mol, 50.9% of the total energy of 36.8603kcal/mol, to 3 and 13.0135kcal/mol, 44.5% of the total energy 29.2475kcal/mol to 4. This shows that the products are experiencing significant ring-strain. On comparison of the energy contributions, it is clear that all energies are slightly lower for 4. The difference in bending energy is large, however. On inspection of the alkene bond angles in the compounds it is clear why 3 is much more unstable. The bond angle in 3 is 107.5o, compared to 112.8o in 4.As a result, the double bond experiencing more ring-strain will be hydrogenated over the other. This means that 4 will dominate, and the reaction is under thermodynamic control.
Stereochemistry & Reactivity of an Intermediate in the Synthesis of Taxol
The isomers 9 and 10 are apparent intermediates in the synthesis of anti-cancer drug Taxol. The
isomers differ in that in 9 the carbonyl group is pointing "up" and in 10 is pointing "down". Using the
MM2 force-field, the more stable isomer was calculated to be 9 with an energy of 47.8396kcal/mol,
compared to 48.1580kcal/mol for 10.

The alkene in these isomers reacts extremely slowly. This is due to the known stability of alkenes situated next to bridgeheads.iii The olefins are said to be "hyperstable" and have negative olefin strains (strain energy less than parent hydrocarbon). This is seen here with the total energy for parent hydrocarbon of 9 being 71.1905kcal/mol and for the parent hydrocarbon of 10, 59.8055kcal/mol. As you can see, the difference in energy between 9 and the parent hydrocarbon is considerably larger than for 10, suggesting that the stability of 9 is greatly increased.
Isomer 9 is more thermodynamically stable than 10. This can be explained by considering the enol tautomers of each isomer. For 9, the two double bonds are situated in such a way that a favourable endo-chair arrangement is formed (see figure). For 10, the slightly higher energy twist-boat conformation is formed.iv
Normal 0 false false false EN-GB X-NONE X-NONE
Regioselective Addition of Dichlorocarbene
Using the MOPAC/PM6 method, the molecular orbitals of compound 12, namely the HOMO, HOMO-1, LUMO, LUMO+1, LUMO+2, can be displayed graphically in order to determine the regioselectivity of attack by dichlorocarbene on either alkene bond to give a cyclopropane.
The first thing to notice about the HOMO of 12, is that the molecular orbital does not show equal spread of electron density between both alkene double bonds. The largest region of electron density is found on the double bond endo to the chlorine atom.

Since this side has the greater electron density, this side will be more susceptible to electrophilic attack from dichlorocarbene. This uneven distribution is due to a slight geometrical distortion in the ring exo to the chlorine atom, caused by a stabilising antiperiplanar interaction between the C-Cl σ* in the LUMO+2 and the exo pi-orbital in the HOMO-1.v
The vibrational spectrum for the dialkene was modelled in Gaussview and showed a strong C-Cl stretch at 689.75cm-1 with an intensity of D=308.66(10^-40esu^2cm^2) and a very weak C=C stretch at 1757.44cm-1 with an intensity of D=8.94(10^-40esu^2cm^2)

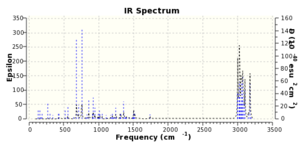
The monoalkene shows a number of vibrations around 1367cm-1 which have increased in intensity compared to the dialkene and correspond to an alkane C-H bending mode. � i C.E. Housecroft & E.C. Constable, Chemistry 3rd ed., 2006, Pearson Education Ltd, 864-5 ii http://www.ch.ic.ac.uk/motm/porphyrins/introDA.html iii A.B. McEwen & P. von Rague Schleyer, J. Am. Chem. Soc., 1986, 108(14), 3951 iv S.W. Elmore & L.A. Paquette, Tetrahedron Lett., 1991, 32(3), 319 v Normal 0 false false false EN-GB X-NONE X-NONE Halton, Boese & Rzepa, J. Chem. Soc., Perkin Trans. 2, 1992, 447-8
