Rep:Module1:scastiglione1
The Basic Techniques Of Molecular Mechanics And Semi-Empirical Molecular Orbital Methods For Structural And Spectroscopic Evaluations
Computational Lab Module 1
S.Castiglione
Introduction
This wiki page contains an investigation into the use of molecular modelling for the purpose of analyzing molecules energetically and in terms of orbitals. Molecular mechanics uses classical mechanics to model a molecular system. The methods used involve the application of Hooke's Law to describe the force field in which atoms move, enabling us to determine to optimum geometry of a given molecule and various analytical values. Whilst these values are not individually definitive they are useful in analyzing different conformers of a given molecule and are useful in comparison. Naturally, the method, like all, has limitations, which are visible when one attempts to apply the information to real chemical problems.The basic analyses in the primary section culminate in the creation of a mini project in which computational chemistry is used to analyse an organic isomer.
Part 1: The Hydrogenation Of Cyclopentadiene Dimer
The dimerisation of cylopentadiene proceeds via a Diels Alder ([4+2] cycloaddition) resulting in a six-memebered ring. The concerted mechanism involved is stereospecific with respect to the dienophile and thus, the stereochemistry is retained in the product. Two stereoisomers are possible, depending on the orientation in which the dieonphile approaches the diene, the endo isomer and the exo isomer. The former is where the substituents on the dienophile are pointing towards the diene, the latter where the substituents are pointing away from the diene.
Molecules 1 and 2
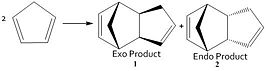
The most thermodynamically stable of molecules 1 and 2 is number 1, the exo dimer as opposed to the endo dimer which is higher in energy, as calculated by the MM2 force field. The relative energies are shown in the table below.
| Exo Dimer (1) | Endo Dimer (2) |
|---|---|
| 31.8949 kcal/mol | 34.0026 kcal/mol |
One knows [1]that the Endo product is preferentially formed in the Diels-Alder reaction. From the relative energies of the two products, as calculated using Molecular Mechanics, one can see that the less stable product is that which predominates. The exo dimer is most likely the more thermodynamically stable molecule as it is less sterically hindered. The endo dimer is is subject to 1,4 steric strain from the hydrogens on the double bond and the pentane ring, making it thermodynamically less stable. The formation of this less stable product indicates that the progress of this reaction is governed by kinetics, as the most theremodynamically stable product is not the one which is formed. This would also indicate that the reaction is most likely irreversible. The reason the endo-product is preferentially formed is likely due to secondary p orbital interactions between the diene HOMO and dienophile LUMO, stabilising the endo transition state with repect to the exo transition state.
Jmol rotatable molecules 1 and 2.
Press the buttons below to open up Jmol files for the molecules.
Molecules 3 and 4

Molecules 3 and 4 are the possible products of hydrogenation of the endo dimer (molecule 2).
The two hydrogenation products, molecules 3 and 4, were simulated and compared with the intention of obtaining a thermodynamic prediction of the relative ease of hydrogenation of the double
| Value | Molecule 3 | Molecule 4 |
|---|---|---|
| Stretch | 1.2369 kcal/mol | 1.0942 kcal/mol |
| Bend | 18.9281 kcal/mol | 14.5450 kcal/mol |
| Stretch-Bend | -0.7619 kcal/mol | -0.5512 kcal/mol |
| Torsion | 12.1748 kcal/mol | 12.4823 kcal/mol |
| Non-1,4 VDW | -1.5462 kcal/mol | -1.0799 kcal/mol |
| 1,4 VDW | 5.7372 kcal/mol | 4.5278 kcal/mol |
| Dipole/Dipole | 0.1631 kcal/mol | 0.1407 kcal/mol |
| Total Energy | 35.9320 kcal/mol | 31.1589kcal/mol |
bonds in the endo product of cyclopentane dimerisation, molecule 2. MM2 calculations were performed and as one can see in the table to the left, product 4 is more stable than product 3. This comparative stabilisation would suggest that the reduction of the cyclopentadiene dimer proceeds first with hydrogenation of the six-membered ring double bond, followed by hydrogenation the five-membered ring. It is known that under mild conditions, one dihydro product is formed and after a more prolonged time the tetrahydro product is formed. This initial faster step suggests that the primary stage of the reaction is likely governed by kinetics prior to the formation of the tetrahydro derivative.
The MM2 energy that is calculated is composed of several considerations, as listed in the table to the right, where each individual component energy is associated with the deviation of that function from its ideal value, for example a distortion from an optimal position, as dictated by the positioning of the atoms comprising the molecule.
As one can see from the table, nearly all positive contributions to the total energy (with the exception of torsion) are larger(more positive) for molecule 3 than for molecule 4. Molecule 3 has a larger bending term as the H-C-C bond angle is more deformed from its' optimal 120.0° (actual = 127.0°) whereas in molecule 4 the H-C-C distortion from its' ideal 109.4° is less (actual = 110.4°).The non van der waals attractions and stretch bend attractions are more positive for molecule 4. Overall the energy of molecule 3 is comparatively a considerable amount larger than for molecule 4. This makes molecule 3 the kinetic product and molecule 4 the thermodynamic product. If the step to form the dihydro is in fact, as theorised, kinetically controlled, it will be molecule 3 that is preferentially formed as, whilst the product stability is less than for molecule 4 (as it is less hindered in the thermodynamic sense), the transition state is of a lower energy and thus molecule 3 is more quickly formed. Then with time the second alkene bond is able to be hydrogenated.
Jmol rotatable molecules 3 and 4.
Press the buttons below to open up Jmol files for the molecules.
Part 2: The Stereochemistry Of Nucleophilic Additions To A Pyridinium Ring (NAD+ Analogue)
The examples in this section are those of the optically active derivative of prolinol (Molecule 5) which reacts with methyl magnesium iodide to alkylate the pyridine ring in the 4-position, with the absolute stereochemistry shown in Molecule 6, and in the second example, the pyridinium ring of Molecule 7 is derivatised by reaction with aniline to form Molecule 8 which acts to transfer the NHPhenyl group to electrophiles[2]. This is a reverse of the reaction by which it was formed. The origins of the stereocontrol in the formation of these molecules will be addressed. The absolute energies of these molecules are useful only in the selection of the conformation of lowest energy. The MMFF94 force field option is used to minimise and determine the energy of these molecules.
Molecules 5 and 6

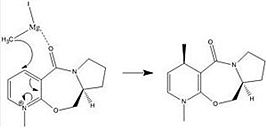
After examination of the conformations of molecule 5, that which is shown in the Jmol file below is of the lowest energy of 58.0288 kcal/mol. Other conformations gave energies ranging between 59 and 65 kcal/mol, including that with the oxygen atom in the opposite direction to that neccesary to give the indicated in the schematic. In molecule 5 the angle between the plane of the six membered ring and the carbonyl is 143.6o, for molecule 6 it is 0o,i.e it is in the plane of the ring. One might expect that the energy of the molecule will differ with the dihederal angle of the carbonyl relative to the plane.
The absolute stereochemistry exhibited in molecule 6 is due to the mechanism of the nucleophilic addition of the grignard reagent MeMgI to the pyridinium ring, as shown to the right[3]. This reaction has high stereo and regioselectivity as the MeMgI methyl will only add to the pyridinium ring at the 4 position relative to the nitrogen atom, and always attacks on the top face of the ring. There is very little flexibility in the larger ring and repeated optimisation of molecule 5 shows that it is not possible for the amide carbonyl to be positioned below the pyridinium ring. The selectivity is because the electrostatic interaction between the carbonyl oxygen and the magnesium of the other reagent, two hard species, allows coordination rather than bond formation, and subsequently, the methyl group is in a position, enhanced by the stereochemistry of molecule 5, where it is able to add to the ring. As the amide carbonyl cannot be positioned below the pyridinium ring, the product of the reaction can only exist with the methyl group pointing upwards. Unfortunately if the MeMgI is included in the optimisation, the program returns an error message. MM2 is able to handle the Mg but the appropriate force field parameters are necessary and I do not currently have said parameters.
Jmol rotatable molecules 5 and 6.
Press the buttons below to open up Jmol files for the molecules.
Molecules 7 and 8
| Molecule 7 | Molecule 8 |
|---|---|
| 99.6465 kcal/mol | 47.8194 kcal/mol |
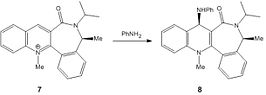
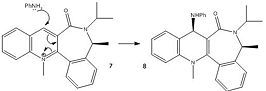
Unlike in the mechanism for the formation of molecule 6, there is no coordination to the oxygen as the nitrogen lone pairs attack directly, rather than the more indirect addition of the methyl group, as seen in the formation of molecule 6 from molecule 5. The given molecule's rigidity due to the fused rings means that only a limited number of conformations are possible. In the minimised version of molecule 7, the oxygen is 20° out of the plane if the conjugated ring system, far less distorted than for molecule 5, and thus, here, the lone pairs on the nitrogen actually repel the lone pairs on the oxygen. This is especially prevalent as both the nucleophile and the carbonyl species are electron rich. The result of this is that the attack is less sterically hindered on the face opposite to that the nitrogen is on, and so it is to this face it adds. The MMFF94 force field option was used to determine the energies of molecules 7 and 8 which are shown in the table to the left, being 99.6465 kcal/mol and 47.8194 kcal/mol respectively in their lowest energy conformations. The MM2 and MMFF94 approximations are basic models that account for sterics, dipole forces and basic lone pair interactions. The possibility of orbital overlap or conjugation is not accounted for, and whilst it might not be a large factor in this section of the module, it is possible that in other sections it will become an issue.
Jmol rotatable molecules 7 and 8.
Press the buttons below to open up Jmol files for the molecules.
Part 3: Stereochemistry And Reactivity Of An Intermediate In The Synthesis Of Taxol
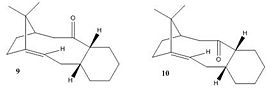
Molecules 9 and 10 are atropisomers of a key intermediate in the total synthesis of Taxol, a drug for the treatment of ovarian cancer. In molcule 9 the carbonyl group is said to be pointing 'up', in molecule 10 it is said to be pointing 'down', as can be visualised from the diagram to the right or the Jmol files below. An atropisomer is a stereoisomer resulting from hindered rotation about a single bond, where the steric strain barrier to rotation is high enough to allow for the isolation of a single conformer.[4] In this particular example, upon standing, the compound isomerises to just one of the possible confomers, that which is most stable, and it has been noted[5] that during subsequent functionalisation of the alkene, the said reaction occured unusually slowly. The MM2 force-field function will be used to determine the most stable of the isomers, molecules 9 and 10, and to rationalise why the alkene reacts so slowly. It is important when attempting to minimise the energies of a conformer to ensure that the molecule is at it's true minimum, and not a local minima, caused by only small perturbations of the molecule. Therefore many different starting points must be used.
Molecules 9 and 10
| Value | Molecule 9 (MM2) | Molecule 10 (MM2) |
|---|---|---|
| Stretch | 2.6746 kcal/mol | 2.5501 kcal/mol |
| Bend | 15.8527 kcal/mol | 10.7196 kcal/mol |
| Stretch-Bend | 0.3928 kcal/mol | 0.3242 kcal/mol |
| Torsion | 18.2484 kcal/mol | 19.6325 kcal/mol |
| Non 1,4 VDW | -1.0730 kcal/mol | -1.3260 kcal/mol |
| 1,4 VDW | 12.6581 kcal/mol | 12.5579 kcal/mol |
| Dipole/Dipole | 0.1478 kcal/mol | -0.1812 kcal/mol |
| Total Energy | 48.9014 kcal/mol | 44.2771 kcal/mol |
| Value | Molecule 9 (MMFF94) | Molecule 10 (MMFF94) |
|---|---|---|
| Total Energy | 70.5498 kcal/mol | 60.5661 kcal/mol |
For these molecules, it was important to ensure they remained in the chair conformation to minimise the energies. It is not surprising to find that molecule 10 is lower in energy in both force field approximations than molecule 9. The MM2 and MMFF94 calculations may appear to give drastically different results; this is quite normal as calculations done with different force fields cannot be compared. One can only compare conformers of the same molecule calculated in the same force field. The important factor is that molecule 10 is of lower energy in both. In molecule 10, the carbonyl groups and the bridging methyl groups are furthest apart so the steric clash is lessened. In molecule 9 these functionalities are forced into close proximity creating considerable unfavourable steric strain and thus higher instability. As it can be seen from the table of MM2 calculations, the torsion contributes the most energy to both molecules, contributing about 18 kcal/mol. The dihedral angle about the carbonyl is different in molecules 9 and 10 (21 and 43 degrees respectively). The larger angle in molecule 10 means the torsion strain is less than for molecule 9. It is still considerable however as the optimum angle would be much larger.
Olefin strain energy (OSE) is defined as the difference between the strain energy of an olefin and the corresponding saturated hydrocarbon[6]. Molecules 9 and 10 are more unreactive than one might expect. This is because they are what is referred to as 'hyperstable olefins'; molecules where the alkene is less strained than the hydrogenated alkane, i.e the OSE is negative. This unusual property can sometimes add to the stability of the molecule where the energy comes from the alkene adjacent to the bridgehead. The frontier molecules are farther away from each other due to the steric clash that would be felt by the carbon attached to the bridgehead. The HOMO-LUMO gap decreases as the orbitals are distorted from being perpendicular to the carbon-carbon double bond. The transition states leading to addition products would therefore be extremely strained and unfavourable to form.[7]
Jmol rotatable molecules 9 and 10.
Press the buttons below to open up Jmol files for the molecules.
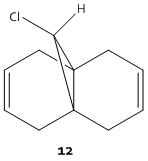
Part 4:Modelling Using Semi-Empirical Molecular Orbital Theory; Regioselective Addition Of Dichlorocarbene (Including Optional Extra Molecules)
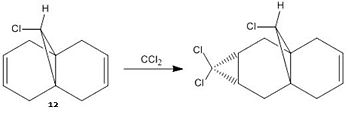
The molecular mechanics approach is limited as it is a purely mechanical model and does not account for secondary orbital interactions. The semi-empirical molecular theory is a different approach whereby electronic aspects of reactivity can be seen. This method considers the probability of electron distribution within the molecule and the effects this distribution will have, on, for instance, spectroscopic properties and bond lengths. The electrophilic addition of dichlorocarbene [8](and other similiar elecrophilic reagents) to molecule 12 is a good example of how these orbital effects control reactivity. For this kind of reaction, in order to model it, one must first optimise the geometry of molecule 12. This optimisation is carried out by first using the MM2 force field method, and then the MOPAC/PM6 method. The PM6 method is used to gain an approximate representation of the valence-electron molecular wavefunction.
Molecule 12
| Orbital | Visualisation | Orbital | Visualisation | Orbital | Visualisation | Orbital | Visualisation | Orbital | Visualisation |
|---|---|---|---|---|---|---|---|---|---|
| LUMO + 2 |  |
LUMO + 1 |  |
LUMO |  |
HOMO | 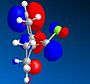 |
HOMO - 1 | 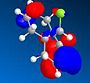
|

Molecular Orbitals
The MM2 and PM6 optimisations lead to very similar conformations with minimal differences between them. The orbital investigation following the PM6 optimisation shows that the orbital most vulnerable to electrophilic attack is the HOMO. The HOMO distinguishes between the two alkene bonds. The alkene bond endo to the Cl substituent is the most nucleophilic and would allow attack from the less hindered face opposite the cyclopropyl ring. The other alkene functionality, that which is exo to the Cl substituent has a region of depleted electron density.
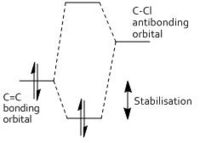
In the HOMO one can see that there is significantly more electron density on the double bond syn to the C-Cl bond than on the other double bond. The distance between either of the exo double bond carbons and the central bridgehead carbon are shorter than the corresponding endo distance. This is a result of an antiperiplanar stabilisation. This is induced by the C-Cl σ* [LUMO+1] orbital interacting with the exo π [HOMO-1] orbital. The π orbital donates electron density into the antibonding C-Cl orbital. [9]This donation into an antibonding orbital weakens the C-Cl bond but it also lowers the energy of the molecule.
That electron density is donated from the exo π orbital explains the observed selectivity of the endo double bond by electrophiles.The HOMO and HOMO-1 display the largest regions of electron density, thus these are most susceptible to electrophilic attack. As they are positioned over a double bond, a functionality known to be electron rich, this is non an unusual observation. A nucleophile however would be most likely to form an SN2 attack into the LUMO+1 near the weakened C-Cl bond as this orbital is both large and empty.
Vibrations
| IR Spectra | Vibration | Image | Vibration | Image |
|---|---|---|---|---|
| C-Cl 774.94cm-1 |  |
C=C 1758.05 cm-1 |  |
| Molecule | Minimised Energy |
|---|---|
| 12 | 17.9014 kcal/mol |
| 12.1 | 22.3592 kcal/mol |
| 12.2 | 17.6565 kcal/mol |
| 12.3 | 16.7237 kcal/mol |
| 12.4 | 17.6088 kcal/mol |
| 12.5 | 15.5811 kcal/mol |
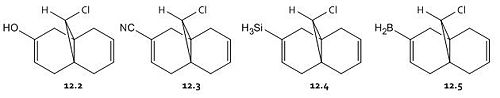
The main objective of this section is to examine the effect on the vibrations of molecule 12 when the carbon-carbon double bond anti to the chlorine is replaced with a single carbon-carbon bond. This is done in order to calculate the influence of the Cl-C bond on the vibrational frequencies of the molecule. In each case the C=C and C-Cl stretches will be observed. This alteration changes the symmetry of the molecule from the original Cs symmetry. With this replacement, one would expect the pi orbitals of the said bond to be no longer filled, thus the electron density of this bond decreases and the result would be a stronger C-Cl bond. The reason for this weakening of the C-Cl bond in the presence of a double bond in the 'anti' position is therefore due to electron density being donated from the double bond into the sigma antibonding orbital of the C-Cl bond.
The C-Cl vibrational stretches appear at c. 770 cm-1 whilst C=C vibrational stretches appear at c. 1700cm-1. A higher vibrational frequency (higher wavenumber (cm-1)) is characteristic of a stronger bond. For molecule 12 the C-Cl frequency is at 770.84cm-1, the C=C anti to the chlorine is at 1737.06cm-1,and the stretching vibration for the C=C syn to the chlorine is at 1757.45cm-1. As expected, there is only one C=C vibration in the monoalkene, at 774.94 cm-1, and the C-Cl stretch is shifted to 1758.05 cm-1, indicative of a stronger C-Cl and C=C (syn) bond, for the reasons described above. This strengthening of the C=C syn bond, thus strengthening the alkene, increasing the electron density of the said bond, makes the alkene more reactive. This increased reactivity would make the alkene less stable, so one might expect the energy of the molecule to be higher. An MM2 calculation of the energies of each molecule was carried out. Whilst all the dialkene species are of 15-17 kcal/mol, the monoalkene has an energy of 22.3592 kcal/mol, in conjunction with the theory.
By modifying the substituents on the anti alkene we effect the Cl-C and C=C frequencies. The substituents -OH, -CN, -SiH3 and -BH2 were added to the anti alkene and the geometries optimised. Upon addition of the substituents the C-Cl frequency decreased and thus the strength of this bond decreases. One might expect that some of the electron density in the molecule has become more localised over the area of the substituents. The stretches of all the anti carbon-carbon double bonds, with the exception of that bearing the -OH decrease and the stretches of all the syn carbon-carbon double bonds decrease, but only very very slightly. It is not surprising that the syn bond is less affected as it is the C-Cl sigma antibonding - C=C anti pi bonding orbitals' interaction that is most prevalent. Thus as the strength of the C-Cl bond decreases, the strength of the anti C=C bond decreases also. This weakening makes the molecule less reactive and thus more stable, and according to MM2 calculations, the energy of all the substituted molecules decreases.
Jmol rotatable molecules 12, 12.1, 12.2, 12.3, 12.4 and 12.5
Press the buttons below to open up Jmol files for the molecules.
Published Optimisation Files;
Molecule 12Molecule 12.1Molecule 12.2Molecule 12.3Molecule 12.4Molecule 12.5
Structure Based Mini Project Using DFT-Based Molecular Orbital Methods
Many synthetic reactions give mixtures of products, some of which will be isomers. If one has isolated and/or separated these isomeric products, it is important to know which isomer(s) have been formed. Sometimes an understanding of the mechanism of the reaction will allow us to predict which isomer will predominate but it is still necessary to confirm that the expected product has been obtained, and if more than one isomer is produced, to be able to say for sure which-is-which. Only certain products can be purified and analysed by X-ray crystallography so techniques like mass spectroscopy, NMR, UV and IR spectroscopy are commonly used in the determination of structure. For determining stereochemistry, extremely useful information can be obtained from 3-bond 1H J values, since these depend on the dihedral angle between the two protons according to the karplus equation, and molecular modelling can be used to predict dihedral angles (and thus the J values).
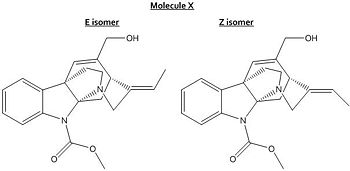
In this mini project I aim to compare the experimental and computational data for the E and Z isomers of molecule X, a clear colourless oil, taken from the primary literature [10] . The data given specifies the E isomer. I hope to be able to correlate my data for the E isomer with the literature and observe differences with the Z data. Molecule X is very rigid so very little transition between the two isomers should occur.
E and Z Isomers Of Molecule X
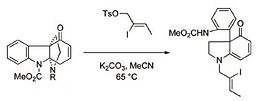
The synthesis of molecule X is complicated and has many steps, but the important step here with respect to the sterochemistry of the alkene is shown to the right (taken from the primary literature[11]). As one can see, the stereochemistry of molecule X depends on the stereochemistry of the iodo-tosyl-alkene used. In the literature, the E alkene was used and so the E isomer of X is produced. If a racemic mixture or unknown isomer of the said molecule was used in synthesis it would be necessary to analyse the products to deduce the isomers at the end.
The isomers were optimised using the density functional theory approach, specifically the B3LYP 6-31G(d,p) procedure. The energies of the molecules were calculated to be 31.2134 kcal/mol for the E isomer and 35.1887 kcal/mol for the Z isomer which are quite similar and suggest the E isomer is more stable.
All experimental data below is from the primary sources.[12]
NMR Spectra
The NMR solvent used was d-benzene for all calculations.
13C NMR Spectra
| Type | E Literature (ppm) | E (no.) | E shift (ppm) | E Deviation(ppm) | Z (no.) | Z shift (ppm) | Z Deviation (ppm) | Difference Between E and Z [E-Z] (ppm) |
|---|---|---|---|---|---|---|---|---|
| Alkene CH3 | 154.2 | 23 | 181.6 | 27.4 | 22 | 180.9 | 26.7 | 1 |
| Cyclohexane Ring CH2 | 144.6 | 14 | 164.5 | 19.9 | 13 | 164.9 | 20.3 | 0.4 |
| Cyclohexane Ring - Bridging CH | 140.7 | 15 | 160.8 | 20.1 | 12 | 153.5 | 12.8 | 7.3 |
| Bridging CH2 | 137.7 | 13 | 153.2 | 15.5 | 14 | 149.9 | 12.2 | 3.3 |
| Ether CH3 | 134.0 | 27 | 145.2 | 11.2 | 16 | 148.0 | 14.0 | 2.8 |
| Bridging CH2 | 128.4 | 12 | 143.4 | 15.0 | 11 | 142.2 | 13.8 | 1.2 |
| Bridging CH2 | 122.6 | 17 | 140.4 | 17.8 | 26 | 142.1 | 19.5 | 1.7 |
| Cyclohexane- Bridging- Cyclopentane C | 122.4 | 2 | 139.0 | 16.6 | 1 | 138.3 | 15.9 | 0.7 |
| CH2 Next To OH | 121.2 | 21 | 131.7 | 10.5 | 20 | 126.4 | 5.2 | 5.3 |
| Cyclopentane C next to both N's | 119.5 | 6 | 102.7 | 16.8 | 5 | 102.0 | -0.7 | 17.5 |
| Benzene Ring CH | 116.0 | 10 | 84.9 | -31.1 | 9 | 86.3 | -29.7 | -1.4 |
| Alkene CH | 92.1 | 20 | 78.7 | -13.4 | 19 | 78.4 | -13.7 | 0.3 |
| Benzene Ring CH | 65.5 | 8 | 78.1 | 12.6 | 7 | 77.5 | 12 | 0.6 |
| Benzene Ring CH | 56.2 | 7 | 77.1 | 20.9 | 6 | 76.3 | 20.1 | 0.8 |
| Cyclohexane Alkene CH | 53.0 | 19 | 74.7 | 21.7 | 8 | 72.0 | 19 | 2.7 |
| Benzene Ring CH | 52.9 | 9 | 72.6 | 19.7 | 18 | 68.0 | 15.1 | 4.6 |
| Benzene-Cyclopentane C | 51.8 | 3 | 63.5 | 11.7 | 2 | 64.0 | 12.2 | -0.5 |
| Alkene C | 38.5 | 16 | 63.2 | 24.7 | 17 | 60.9 | 22.4 | 2.3 |
| Benzene-Cyclopentane C next to N | 31.1 | 4 | 60.1 | 29.0 | 3 | 60.3 | 29.2 | -0.2 |
| Cyclohexane Alkene C | 28.1 | 18 | 58.4 | 30.3 | 15 | 58.8 | 30.7 | -0.4 |
| Ether C between 2 O's and N | 14.3 | 24 | 50.2 | 35.9 | 23 | 49.0 | 34.7 | 1.2 |
At the extremes of the spectra the data correlates less well than towards the middle. As no assignments were made in the literature I have gone by the assignments produced by Gaussian. There is a slight difference between the E and Z spectra with E being at slightly higher shifts than Z. The difference would not be enough to tell an isomer from a single spectra but perhaps if you had two spectra you could tell which was which, i.e if you managed to isolate the two isomers but didn't know which one was which you might be able to use Carbon 13 NMR.
1H NMR Spectra
I attempted to calculate the 1H NMR Spectra for the compounds however all the shifts ranged between 24 and 32 ppm, way out of line with the literature which places all the peaks within 1 to 9 ppm. To calculate the hydrogen NMR was not suggested in the script and has obviously not worked. Perhaps despite the option to read off the hydrogen NMR, it is not truly a feasible option for this method. The multiplicities also do not match the experimental data as all lines are singlets and the maximum integral is two. Nonetheless I have presented the obtained spectra here and the literature data is listed below.
1H NMR (500 MHz, C6D6, 60°C)[8.08 (br s, 1H), 7.14 (m, 1H), 6.94 (s, 1H), 7.06 (d, J = 7.4 Hz, 1H), 6.89 (m, 1H), 5.67 (s, 1H), 5.16 (q, J =6.8 Hz, 1H), 4.22 (br s, 1H), 3.92 (q, J = 13.5 Hz, 2 H), 3.57 (s, 3H), 3.23 (br s, 1H), 2.94−2.99 (m, 2H), 2.51−2.56 (m, 2H), 1.87 (ddd, J = 12.4, 7.1, 7.1 Hz, 1H), 1.59−1.61 (m, 4H), 1.52 (dd, J = 12.1, 5.6 Hz, 1H)][13]
IR Spectra
 E Isomer IR Spectrum |
 Z Isomer IR Spectrum |
| Literature IR of E Isomer | Calculated IR of E Isomer | Calculated IR of Z Isomer | Assignment |
|---|---|---|---|
| - | 3804 | 3778 | OH Stretch |
| 3994 | 3205 | 3207 | Benzene CH Stretch |
| - | 3000-3200 | 3000-3200 | CH3/CH2 Stretches |
| - | 1803 | 1825 | CO Stretch |
| 1697 | 1657 | 1657 | Ph Stretch |
| - | 1300-1550 | 1300-1550 | CH3/CH2 Bends |
The literature IR data was for the neat liquid, was unassigned, and only quoted two bands. I have assigned these bands and others found from the calculations. As with the carbon NMR the data for both isomers only differs a little and it might be possible to distinguish them if two spectra were side by side but if one had only a single spectrum it would be very difficult to tell what isomer it was from.
Optical Rotation
I have not been able to run an optical rotation as each file I have tried to run has taken too long and has timed out.
The literature[14] gives several optical rotations for the E (+) isomer for different wavelengths, and one would assume the Z (-) isomer would give the same, but negative, values. This may in fact be the best way to distinguish which isomer you have as the values are high. The values given are as following; (Format: [α]wavelength(nm) optical rotation (degrees) at a concentration of 0.9M in CHCl3) [α]589 +15.9, [α]577 +16.5, [α]546 +19.8, [α]435 +46.1, [α]405 +63.6
I have several more optical rotation files currently attempting to run (although they have been running for several days as it is) and if any of these succeed I will endeavour to update this page as soon as possible, even if it is after the due date (I will send an update email). The job numbers for these current jobs and the date initiated are 24869 (28/2/10), 24870 (28/2/10), 24984 (01/3/10), 24985 (01/3/10), 24986 (01/3/10), 24987 (01/3/10). The failed jobs are listed in the published files section. This is unfortunate as I believe the optical rotation would actually probably be the most effective distinction method.
The optical rotation files were run at 589 nm and so I would expect the values returned to be similar to [α]589 +(E)/-(Z) 15.9
Jmol rotatable molecules for the E and Z isomers of molecule X.
Press the buttons below to open up Jmol files for the molecules.
Published Optimisation Files;
E Isomer OptimisationZ Isomer Optimisation E Isomer IRZ Isomer IR E Isomer 13CZ Isomer 13C
Failed Optical Rotation Files;
E Isomer Optical RotationZ Isomer Optical Rotation
Conclusion
In conclusion, optical rotation would probably, in this case, be the best way to distinguish between the two isomers. The other methods would only be of real use if one had a spectra for each isomer to compare.
References
- ↑ J. Clayden, N. Greeves, S. Warren and P. Wothers, Organic Chemistry, Oxford University Press, 2001, 916
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
- ↑ S. Leleu, C.; Papamicael, F. Marsais, G. Dupas, V.; Levacher, Vincent. Tetrahedron: Asymmetry, 2004, 15, 3919-3928. DOI:10.1016/j.tetasy.2004.11.004
- ↑ Bringmann G, Mortimer AJP, Keller PA, Gresser MJ, Garner J, Breuning M (2005). "Atroposelective Synthesis of Axially Chiral Biaryl Compounds". Angewandte Chemie International Edition 44 (34): 5384-5427.doi:10.1002/anie.200462661
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- ↑ Borden, W. T. Chem. Rev. 1989, 89, 1095
- ↑ Wilhelm F. Maier, Paul Von Rague Schleyer -Journal of the American Chemical Society 1981 103 (8), 1891-1900
- ↑ B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P29920000447
- ↑ Brian Halton and Sarah G. G. Russell J. Org. Chem. 1991,56,5553-5556>Brian Halton and Sarah G. G. Russell J. Org. Chem. 1991,56,5553-5556
- ↑ Dounay, Amy B.; Humphreys, Philip G.; Overman, Larry E.; Wrobleski, Aaron D.; JACSAT; Journal of the American Chemical Society; English; 130; 15; 2008; 5368 - 5377; DOI: 10.1021/ja800163v; ISSN: 0002-7863
- ↑ Dounay, Amy B.; Humphreys, Philip G.; Overman, Larry E.; Wrobleski, Aaron D.; JACSAT; Journal of the American Chemical Society; English; 130; 15; 2008; 5368 - 5377; DOI: 10.1021/ja800163v; ISSN: 0002-7863
- ↑ Dounay, Amy B.; Humphreys, Philip G.; Overman, Larry E.; Wrobleski, Aaron D.; JACSAT; Journal of the American Chemical Society; English; 130; 15; 2008; 5368 - 5377; DOI: 10.1021/ja800163v; ISSN: 0002-7863; Dounay, Amy B.; Overman, Larry E.; Wrobleski, Aaron D.; JACSAT; Journal of the American Chemical Society; English; 127; 29; 2005; 10186 - 10187; ISSN: 0002-7863.
- ↑ Dounay, Amy B.; Humphreys, Philip G.; Overman, Larry E.; Wrobleski, Aaron D.; JACSAT; Journal of the American Chemical Society; English; 130; 15; 2008; 5368 - 5377; DOI: 10.1021/ja800163v; ISSN: 0002-7863
- ↑ Dounay, Amy B.; Humphreys, Philip G.; Overman, Larry E.; Wrobleski, Aaron D.; JACSAT; Journal of the American Chemical Society; English; 130; 15; 2008; 5368 - 5377; DOI: 10.1021/ja800163v; ISSN: 0002-7863