Rep:Module1:organicwm207
This page aims to answer the questions posed in the Organic Module for the Chemistry department wiki, as part of a third year undergraduate assessed lab course.
Molecular Mechanics
Computational Chemistry relies heavily on this technique as it is one of the more straightforward and less computationally demanding methods of determining simple spatial arrangement of molecules. Molecular Mechanics uses set data about common elements to model molecules based on each element's preferred orientation and a measure of elasticity determining how far it will stray from this orientation (among other methods). The program then sets about manipulating the molecule to relieve as much mechanical strain as possible.
Cyclopentadiene
Dimerisation of Cyclopentadiene
Cyclopentadiene dimerises readily at room temperature to form dicyclopentadiene (DCPD) with specific formation of the endo-dimer. Both the endo- and exo- dimers were sujected to MM2 analysis with the results below.
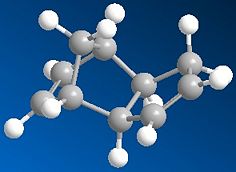 |
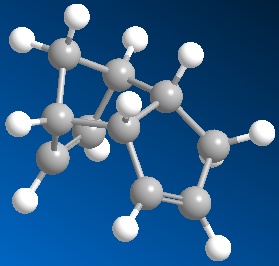 |
| exo-product | endo-product | |
| Stretch | 1.2795 | 1.2474 |
| Bend | 20.8795 | 20.8621 |
| Stretch-Bend | -0.8373 | -0.8340 |
| Torsion | 7.6724 | 9.5064 |
| H-bond | -1.4273 | -1.5387 |
| VdW | 4.2389 | 4.3096 |
| Dipole | 0.3779 | 0.4467 |
| Total | 31.8836 | 33.9994 |
Although the endo-product is lower in energy in some aspects its total energy is actually higher than that of the exo-product, which begs the question why is it formed with preference to the exo product? The explanation is that although the exo-product is indeed lowest in energy, the transition state required to reach it is much higher in energy than the transistion state to reach the endo-product. This is an example of kinetic vs. thermodynamic reaction control. The endo-dimer is the kinetic product, which is formed first, quickest and selectively because the system is not being heated or given any other energy sufficient to overcome the thermodynamic transition state.
Hydrogenation of Cyclopentadiene
Similarly, by comparing the products of hydrogenation at each of the double bonds present in DCPD we can determine which double bond it is more favourable to hydrogenate.
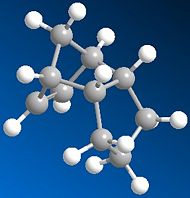 |
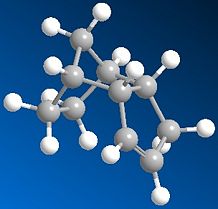 |
| Dimer 3 | Dimer 4 | |
| Stretch | 1.2347 | 1.1048 |
| Bend | 18.9581 | 14.5389 |
| Stretch-Bend | -0.7605 | -0.5474 |
| Torsion | 12.1245 | 12.5103 |
| Non 1,4 VdW | -1.5178 | -1.072 |
| 1,4 VdW | 5.7302 | 4.4956 |
| Dipole | 0.1631 | 0.1407 |
| Total | 35.9323 | 31.1709 |
It is immediately clear that the monoalkene 'Dimer 4' is lower in relative energy and should, in theory be hydrogenated first.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
Prolinol Derivative
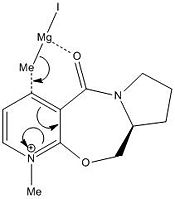
|
The optically active derivative of prolinol reacts with methyl magnesium iodide (a grignard reagent) to alkylate the pyridinium ring specifcally on the top face (as shown in the diagram). The reason for this, as determined by molecular modelling, is that the carbonyl group on the neighbouring ring points up when the molecule is optimised for lowest energy. If the grignard reagent approaches from the top face, the alkylation transition state can be stabilised by favourable interactions between the grignard magnesium and the adjacent carbonyl oxygen. If it approaches from the bottom face there is no favourable interaction and the reaction is subsequently harder to drive forwards.
It is not possible to analyse the transition state directly with Molecular Modelling, in part beacuase the software package (ChemBio 3D) does not contain information for Magnesium, but also because the method only deals with definite bonds and cannot express half-formed bonds, let alone mere favourable coulombic interactions, and as such this is one of its most notable failings as a method.
However, when comparing the product (6) with the reactant (5) it is notable that the angle of the carbonyl above the plane of the ring is markedly decreased for the product, a dihedral angle of 7.8 degrees vs. 22.9 degrees.
 |
Nucleophilic Addition of Aniline
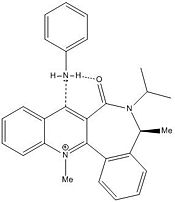
|
The reagent molecule (7) in this example also shows the carbonyl neighbouring the reaction site to be pointing up relative to the aromatic plane when the molecule is optimised using MM2. On this occasion the stereospecificity is explained by the ability of the carbonyl oxygen to hydrogen-bond to the hydrogen on the nucleophilic amine which will increase the nucleophilicity of the amine and aid in the eventual abstraction of the hydrogen altogether. As before it is not possible to model the transition state but again it is notable that the dihedral angle of the carbonyl with the plane of the ring is significantly changed in the product (18.9 degrees for the reagent vs. -14.1 degrees for the product), so much so in fact that it is actually forced to the other side of the plane.
 |
Stereochemistry of a Taxol Intermediate
This molecule presented more of a challenge in terms of directing the caluclation to find the local optimisation of the molecule, in terms of up or down orientation of the carbonyl group on the macrocycle. It is also noteworthy that the cyclohexane ring did not immediately adopt the chair conformation and it was necessary to manipulate it towards the correct conformer.
 |
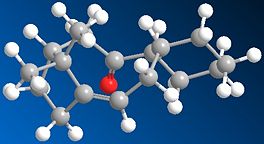 |
| Up | Down | |
| Stretch: | 2.6575 | 2.7629 |
| Bend: | 15.8557 | 15.9462 |
| Stretch-Bend: | 0.385 | 0.3531 |
| Torsion: | 18.2558 | 17.4516 |
| Non-1,4 VDW: | -1.0766 | 0.0672 |
| 1,4 VDW: | 12.6627 | 12.8095 |
| Dipole/Dipole: | 0.1463 | 0.0649 |
| Total Energy: | 48.8863 | 49.4553 |
However, once the data has been calculated it becomes immediately clear that the 'Up' isomer is the more stable, although there's very little difference between them.
Semi-empirical DFT and Molecular Orbital (MO) Theory
This section will use several new methods, including MOPAC (a fast method of approximating MOs), and much more computationally demanding Density Functional Theory approach which gives a much more qualitative understanding of electronic density and allows for analysis of stretching frequencies (IR spectroscopy) as well as NMR data.
Dichlorocarbene
The origins of the regioselectivity of the electrophilic carbenylation of a chloro-substituted bicylcic diene
A fast-running MOPAC calculation was performed for a dichlorocarbene molecule, with results returned in under 30 seconds. The calculated MOs were then overlaid onto the molecule to show exactly where electron density could be found, most importantly for the HOMO/LUMO interface to show where reactivity would be most likely to take place.
| HOMO | LUMO | LUMO+1 | LUMO+2 |
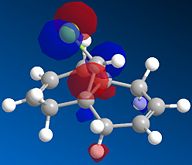 |
 |
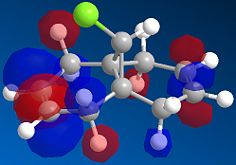 |
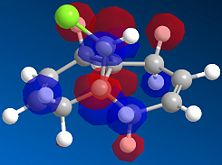 |
Here, the Highest Occupied Molecular Orbital (HOMO) quite clearly shows the most reactive electron density to be centered around the chlorine atom, which is what one would expect. If we were to consider nucleophilic attack on one of the alkene bonds, the LUMO shows us that the Carbon-Carbon double bond anti to the chlorine provides the Lowest Unoccupied Molecular Orbital, into which a nucleophile could donate its electrons to initiate a reaction. The other double-bond is the focus of the LUMO+1, the next highest energy vacant orbital. So although they are both available to nucleophilic attack, it would predominate at the anti-alkene due to the availability of the lower energy molecular orbital.
The use of DFT MO Theory to investigate Neighbouring Group Participation (NGP) on the C-Cl and/or C=C stretching Frequency of the above bicyclic diene
Density Functional Theory calculations take much longer to run than the previous calculations, so it was necessary to submit them to run as jobs on a departmental supercomputer. The molecules being examined were the Dichlorocarbene (from the previous example) and its monohydrated analog. Gaussview was used to define the symmetry of the dialkene as this has the effect of approximately halving the run-time, although this was not possible for the mono-alkene as it does not share the same symmetry (a caveat of being mono-hydrated). Gaussian input files were created and run, the dialkene in ~30 mins and the mono-alkene in just under one hour. The program had been asked to analyse the stretching frequencies of each molecule, in practice the result is a computer generated IR spectrum.
| Stretch | Di-alkene (cm-1) | Mono-alkene (cm-1) |
| C-Cl | 770.93 | 780.05 |
| C=C (anti to Cl) | 1737.01 | - |
| C=C (syn to Cl) | 1757.32 | 1753.75 |
