Rep:Module1:doushinidecuoqingyiaishangwo
Modelling using Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
At room temperature cyclopentadiene slowly dimerises via the Diels-Alder mechanism in favour of the endo dimer over the endo dimer. Hydrogenation of the endo dimer forms two dihydro derivatives; extensive hydrogenation forms the tetrahydro derivative. Cyclodimerisation and hydrogenation can be due to either thermodynamics (product stability) or kinetics (transition state stability) and by calculating the geometries and energies of the products it can be deduced whether the reactions are thermodynamically or kinetically controlled. The MM2 molecular modelling method in Chembio3D has been used to optimise the geometry and minimise the energy of the molecules.
Exo Dimer, 1
 |
 |
| Energy/kcal mol-1 | |
|---|---|
| Stretch | 1.286 |
| Bend | 20.579 |
| Stretch-Bend | -0.838 |
| Torsion | 7.657 |
| Non-1,4 VDW | -1.417 |
| 1,4 VDW | 4.232 |
| Dipole/Dipole | 0.378 |
| Total | 31.877 |
| Energy/Hartree | 0.051 |
Endo Dimer, 2
 |
 |
| Energy/kcal mol-1 | |
|---|---|
| Stretch | 1.251 |
| Bend | 20.848 |
| Stretch-Bend | -0.836 |
| Torsion | 9.511 |
| Non-1,4 VDW | -1.543 |
| 1,4 VDW | 4.320 |
| Dipole/Dipole | 0.448 |
| Total | 34.000 |
| Energy/Hartree | 0.054 |
Hydrogenated Product, 3
 |
 |
| Energy/kcal mol-1 | |
|---|---|
| Stretch | 1.284 |
| Bend | 19.920 |
| Stretch-Bend | -0.831 |
| Torsion | 10.785 |
| Non-1,4 VDW | -1.255 |
| 1,4 VDW | 5.624 |
| Dipole/Dipole | 0.162 |
| Total | 35.689 |
| Energy/Hartree | 0.057 |
Hydrogenated Product, 4
 |
 |
| Energy/kcal mol-1 | |
|---|---|
| Stretch | 1.097 |
| Bend | 14.506 |
| Stretch-Bend | -0.549 |
| Torsion | 12.498 |
| Non-1,4 VDW | -1.051 |
| 1,4 VDW | 4.513 |
| Dipole/Dipole | 0.141 |
| Total | 31.154 |
| Energy/Hartree | 0.050 |
Discussion
The minimalised energy of the exo dimer, 1, is lower than the energy of the endo dimer, 2. The Diels-Alder reaction to form the exo dimer is therefore thermodynamically controlled and the reaction to form the endo dimer is kinetically controlled. In addition the calculated energy of the transition state of 2 is lower than the energy of the transition state of 1, supporting the fact that the reaction to form 2 is kinetically controlled.
Similarly the energy of the hydrogenated endo product, 4, is lower than the energy of the alternative hydrogenated endo product, 3. The hydrogenation reaction to form 4 is therefore the thermodynamically controlled and the reaction to form 3 is kinetically controlled. Again the calculated energy of the transition state of 3 is lower than the energy of the transition state of 4, supporting the fact that formation of 3 is kinetically controlled. 4 would be the favoured product if the reaction was reversible and 3 would be the favoured product if the reaction was irreversible.
Comparison of the individual energy contributions show that 'bending' energy is the major contributor to the difference in the total energy, and therefore stability, between 3 and 4. The remaining individual energy contributions of 3 and 4 are relatively similar.
Stereochemistry of Nucleophilic Additions to a Pyridinium Ring (NAD+ Analogue)
Two examples of nucleophilc additions to a pyridium ring are investigated here. One is the addition of methyl magnesium iodide to reactant 5, an optically active derivative of prolinol; the alkylated pyridine ring (in the 4-position) is product 6. The other is the addition of phenylamine to reactant 7; the derivatised pyridine ring (in the 4-position) is product 8.
The origin of the stereocontrol in the formation of 8 lies within the stereochemistry of 7 and will be discussed later. The MMFF94 molecular modelling method in BioChem3D has been used to optimise the geometry and minimise the energy of the following molecules.
Note: The absolute stereochemistry of the products and reactants are shown.
Reactant 5 - Product 6
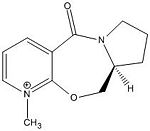 |
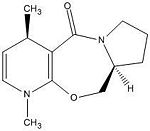 |
The calculated energies are given below.
| Molecule | Energy/kcal mol-1 | Energy/Hartree |
|---|---|---|
| Reactant 5 | 57.387 | 0.091 |
| Product 6 | 6.282 | 0.010 |
| Product 6 Enantiomer | 4.762 | 0.008 |

The are 2 configurations for 5 with the shown stereochemistry. The most optimised geometry of 5 has the carbonyl group coplanar to the aromatic ring. However the other geometry has the carbonyl group above the plane (~36degrees) with respect to the aromatic ring. One would expect methyl magnesium iodide nucleophilic addition to the pyridinium on the 4-position to occur from below the plane as this avoids steric clash between the nucleophile and the carbonyl oxygen. Infact addition from above the plane is favoured because of chelation between the magnesium of the nucleophile and the oxygen of the carbonyl. Since the carbonyl group is above the plane, chelation of the nucleophile to the oxygen means the nucleophile is only able to add from above the plane. Comparison between the energy of 6 (addition above plane) and it's enantiomer (addition below plane) however shows that the enantiomer has a lower energy and is more stable. The reaction to form 6 is therefore kinetically controlled.
Reactant 7 - Product 8
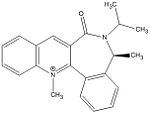 |
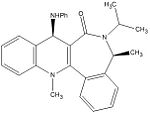 |
The calculated energies are given below.
| Molecule | Energy/kcal mol-1 | Energy/Hartree |
|---|---|---|
| Reactant 7 | 98.633 | 0.157 |
| Product 8 | 36.432 | 0.058 |
| Product 8 Enantiomer | 44.209 | 0.070 |
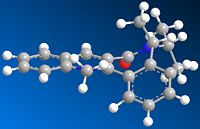
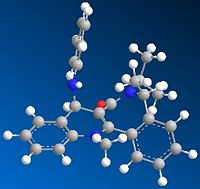
The optimised geometry of 7 has the carbonyl group below the plane with respect to the aromatic ring. Again, one would expect phenylamine nucleophilic addition to occur from above the plane as this avoids steric clash between the nucleophile and the carbonyl oxygen. In this example this is favoured because the nucleophile is very large and would lead to a very high energy transition state if it was to add below the plane. Comparison between the energy of 8 (addition above plane) and it's enantiomer (addition below plane) shows that 8 has the lower energy and is more stable. The reaction to form 8 is therefore thermodynamically controlled.
References
1) A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
2) S. Leleu, C.; Papamicael, F. Marsais, G. Dupas, V.; Levacher, Vincent. Tetrahedron: Asymmetry, 2004, 15, 3919-3928. DOI:10.1016/j.tetasy.2004.11.004
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Paclitaxel, also know as Taxol, is an important drug used in cancer treatment. 9 and 10 are two key intermediates in the synthesis taxol. They are synthesised via carbonyl addition with the carbonyl group pointing either up or down; atropisomerism is present. Which key intermediate will be the major isomer will depend on their relative stabilities.
MM2 and MMFF94 has been used to optimise the geometry and minimise the energy of the following molecules.
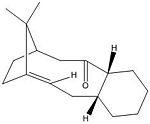
Intermediate 9
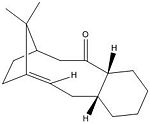
| MM2
Energy/kcal mol-1 |
MMFF94
Energy/kcal mol-1 | |
|---|---|---|
| Stretch | 2.966 | |
| Bend | 18.967 | |
| Stretch-Bend | 0.259 | |
| Torsion | 18.728 | |
| Non-1,4 VDW | -0.073 | |
| 1,4 VDW | 13.641 | |
| Dipole/Dipole | 0.065 | |
| Total | 54.553 | 76.407 |
| Energy/Hartree | 0.087 | 0.122 |
Intermediate 10
| MM2
Energy/kcal mol-1 |
MMFF94
Energy/kcal mol-1 | |
|---|---|---|
| Stretch | 2.503 | |
| Bend | 12.793 | |
| Stretch-Bend | 0.291 | |
| Torsion | 17.721 | |
| Non-1,4 VDW | -0.735 | |
| 1,4 VDW | 12.137 | |
| Dipole/Dipole | 0.257 | |
| Total | 44.967 | 61.028 |
| Energy/Hartree | 0.072 | 0.097 |
Discussion
Geometry optimisation of both intermediates using the MM2 and MMFF94 shows that 10 is the most stable one. Comparison of MM2 and MMFF94 results show very different energies. Intermediate 9 has a relatively higher energy and similar to before 'bend' energy is the major contributar to this difference. This suggests there is possibly more strain in the cyclic structure of intermediate 9 and less rigid.
The alkene of the intermediates reacts slowly because of hyperstability[1][2] of the bridgehead alkene.
References
1) S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319. DOI:10.1016/S0040-4039(00)92617-0
2) J. G. Vinter and H. M. R. Hoffman, J. Am. Chem. Soc., 1974, 96, 5466 and 95, 3051. DOI:10.1021/ja00824a025
3) J. Am. Chem. Soc., 1999, 121, 3226. DOI:10.1021/ja990189i
4)G. ÊIslas-Gonzalez, M. ÊBois-Choussy and J. ÊZhu, Org. Biomol. Chem., 2003, 30-32. DOI:10.1039/b208905
Modelling Using Semi-Empirical Molecular Orbital Theory
In this section we move from a purely classical mechanical treatment of a molecule to a quantum mechanical treatment. We will considerer explicitly the electrons in molecules, and how the electrons influence bonds, derived spectroscopic properties and reactivity.
Regioselective Addition of Dichlorocarbene
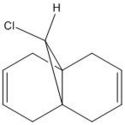
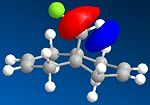
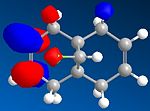
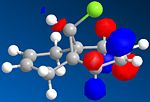
Compound 12 is a dialkene and it reacts with electrophilic reagents such as dichlorocarbene. Which of the two alkenes will dichlorocarbene react with depends on the molecular wavefunction at the alkene, illustrating orbital control of reactivity. In order to illustrate the electronic aspects the geomgetry of 12 needs to be optimised and the energy of the molecular orbitals needs to be calculated displayed.
There are two parts.
Part 1: MM2 is used to optimise the geometry and minimise the energy of 12. MOPAC/PM6 is used to approximate the valence-electron molecular wavefunction including the HOMO, LUMO, LUMO+! and LUMO+2.
Part 2: B3LYP/6-31G(d,p) in Gaussian is used to optimise the geometry and calculate the stretching frequencies of the optimised 12. SCAN (Instructions) was used for quicker calculations.
 |
 |
 |
 |
 |
| Bond | Dialkene
Stretching Frequency/cm-1 |
Monoalkene
Stretching Frequency/cm-1 |
|---|---|---|
| C=C, Cl side | 1757.45 | 1758.05 |
| C=C | 1737.07 | N/A |
| C-Cl | 548.79 | 536.41 |
The SCAN output file can be found here Dialkene Output File and Monoalkene Output File.
Discussion
Compound 12 reacts with dichlorocarbene similarly to an electrophilic addition. Since the size of the substituents, H and cl, on the bridge are relatively small, sterics do not play a major role in determiniing which of the two alkenes will react. We must look at electronic effects in order to determine the more reaction alkene. The HOMO alkene will be the most nucleophilic because the electrons with be concentrated there and hence the alkene will be more reactive with dichlorocarbene. In addition there are antiperiplanar interactions between the C-Cl signa-star orbital and the occupied pie orbiral on the alkene away from the chlorine side which makes it more stable than the alkene on the chlorine side. The method clearly shows discrimination between the two alkenes. MOPAC/PM6 molecular orbital approximation shows the HUMO residing on the alkene on the chlorine side and hence reaction is expected to occur with this alkene. This is supported by the calculated stretching frequencies of the alkenes with the HUMO residing alkene having a higher energy than the other.
The C=C bond on the chlorine side has the same stretching frequency in the diene and monoene. However the C-Cl bond has different streching frenquencies.
References
1) B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P29920000447
Mini Project
This project involes studying a chosen reaction which forms isomeric products. It is important to know which isomer has been formed in isomeric reactions and so spectrometric techniques are required. In this project the GIAO approach is used to predict 13C NMR chemical shift. Other calculations predict 3J H-H coupling constants, IR spectra, optical rotation, and circular dichroism (CD/UV-Visible) spectrum of a compound.
However, many organic compounds are oils or liquids and structure determination relies on spectroscopic methods. Mass spectrometry is a useful starting point, and high resolution mass spectrometry (HRMS) allows the molecular formula to
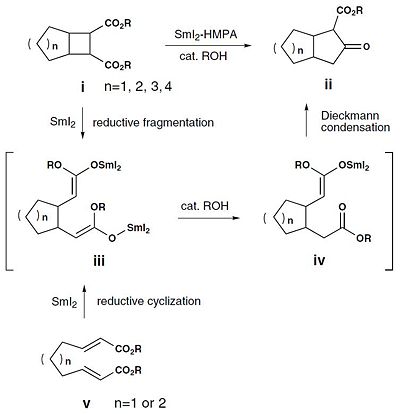
Samarium(II)-induced ring-expansion reaction of 1,2-cyclobutanedicarboxylates to produce cyclopentanones
Dimethyl 1,2-cyclobutanedicarboxylate Reactant
The 13C NMR spectra were predicted, and the chemical shifts were calculated using Gaussian interface in ChemBio3D using the DFT method mpw1pw91/6-31(d,p). Details of the procedure for predicting the 13C NMR spectrum of a compound can be found HERE.
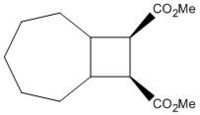
| Carbon | Calculated
Chemical Shift/ppm |
|---|---|
| 9 | 27.4 |
| 6 | 28.3 |
| 8 | 31.4 |
| 7 | 32.6 |
| 5 | 34.2 |
| 3 | 39.4 |
| 1 | 41.2 |
| 2 | 41.4 |
| 4 | 46.9 |
| 17 | 51.0 |
| 13 | 51.3 |
| 10 | 167.3 |
| 14 | 170.2 |

Cyclopentanone Product
There are two isomeric cyclopentanone products possible, 11a and 11b, and these are diastereomers. 1H and 13C NMR, and IR spectroscopy could be used distinguish between the stereoisomers but it won't be very effective. Optical rotation would not be suitable because the isomers are not enantiomers. 13C NMR and IR values have been calculated below and compared to literature values.

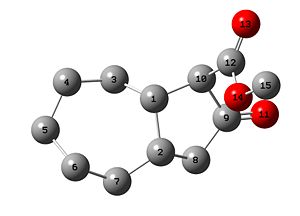
| Carbon | 11a, Calculated
Chemical Shift/ppm |
11a, Reference
Chemical Shift/ppm |
|---|---|---|
| 6 | 26.5 | 26.1 |
| 4 | 32.3 | 29.2 |
| 5 | 32.5 | 31.2 |
| 3 | 33.2 | 32.0 |
| 7 | 34.6 | 32.1 |
| 2 | 40.0 | 38.2 |
| 8 | 46.4 | 43.6 |
| 1 | 47.4 | 47.1 |
| 15 | 51.7 | 52.4 |
| 10 | 66.0 | 61.2 |
| 12 | 165.3 | 169.6 |
| 9 | 209.3 | 211.4 |
| Carbon | 11b, Calculated
Chemical Shift/ppm |
11b, Reference 116
Chemical Shift/ppm |
|---|---|---|
| 5 | 26.9 | 26.6 |
| 4 | 28.6 | 26.7 |
| 6 | 32.0 | 26.9 |
| 3 | 32.3 | 33.5 |
| 7 | 38.4 | 34.2 |
| 2 | 39.1 | 41.0 |
| 8 | 48.0 | 47.3 |
| 1 | 48.7 | 47.4 |
| 15 | 51.7 | 52.4 |
| 10 | 68.4 | 64.2 |
| 12 | 165.4 | 169.5 |
| 9 | 208.8 | 210.5 |
The IR spectrum was predicted, and the vibrational frequencies were calculated using Gaussian interface in ChemBio3D using the DFT method mpw1pw91/6-31(d,p). Details of the procedure for predicting the IR spectrum of a compound can be found HERE.
| Bond | Calculated
Streching Frequency/cm-1 |
Refere ng Frequency/cm-1 | Reference 11b
Streching Frequency/cm-1 |
|---|---|---|---|
| C=O
Ester |
1825 | 1732 | 1732 |
| C=O
Ketone, cyclic 5-membered |
1851 | 1755 | 1757 |
Wikipedia's infrared spectroscopy correlation table [3] was used to correspond the bond type with the literature values. GaussView 3.09 was used to observe the stretching bonds and correspond them to their corresponding frequencies.
Discussion
The majority of the calculated chemical shifts match well with the literature values and are within 2ppm. However the calculated IR values do not match well. The literature states that 11a is the favoured isomer with a product ratio of 1.6:1. Molecular modelling using MM2 however showed that the minimised energy of 11a is higher than the energy of 11b possibly suggesting that the favoured product, 11a, is not a thermpdyanmic product but is kinetically controlled.
References
1) I. Shinohara, H. Nagaoka, Tetrahedron Letters, 7 (45), 2004, 1495-1498 DOI:10.1016/j.physletb.2003.10.071
Additional References
- ↑ A. B. McEwen, P. V. R. Schleyer, J. Am. Chem. SOC. 1986, 108, 3951-3960. DOI:10.1021/ja00274a016
- ↑ W. F. Maier, P. V. R. Schleyer, J. Am. Chem. Soc., 1981, 103 (8), 1891–1900 DOI:10.1021/ja00398a003
- ↑ http://en.wikipedia.org/wiki/Infrared_spectroscopy_correlation_table
4) K. Alder, G. Stein, Liebigs Ann. Chem., 1933, 504, 210-257. DOI:10.1002/jlac.1933504011
Conclusion
The accuracy of the calculated minimum energy can be improved in the MM2 and MMFF94 molecular modelling methods if the minimum RMS gradient was decreased to 0.001 (default 0.1). This would requires more iterations and hence more time to complete the calculations which would be significant for calculations on very large molecules. Also the modelling is limited to only minising the present structure of the molecule. Further minimalisation requires movement of the atoms, rearrange the molecule to the most stable form before optimisation. This is particularly the case for cycloalkanes such as cyclohexane where configuration of the molecule into the chair form would lead to lower energy. Also monosubstituted carbon atoms can be configured where the substituent and a hydrogen can be switched with each other. In general every atom should be as far was way from each other as possible to give th lowest energy.
