Rep:Module1:TomTaylor
The formation and Hydrogenation of Cyclopentadiene Dimers
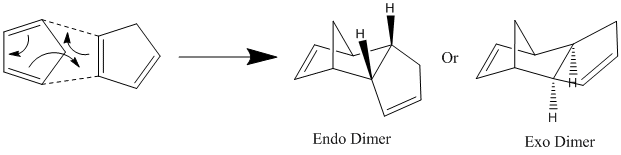
It is known that two molecules of cyclopentadiene can react together in a pericyclic reaction (4 + 2 electrons) to form a dimer. This is shown below:
It can be seen that this reaction the product can either be of the exo form or the endo form. Experimentally it is seen that the endo product predominates. Knowing this fact, it is possible to use a computer simulation to find out whether this reaction is under thermodynamic control or kinetic control. This is done by considering the energy of the two possible products.
After build a model of both the possible products, as shown above in the reaction scheme, it is possible to find the energy of both the products by using a MM2 force field. This is a computer program that considers all the force in a molecule, such as stretching, bending and torsion of the chemical bonds, as well as considering the effects Van der Waals forces and Hydrogen bonding and works out what is the lowest energy for the molecule, balancing all the factors explained above.
It was found that the energy of the exo product (31.88 Kcal/mol or 133.39KJ/mol) was lower than the energy of endo product (34.01 Kcal/mol or 142.30 KJ/mol). As it is known that it is the high energy endo product that is the major product of this reaction, the MM2 force field calculation suggests that this reaction is not under thermodynamic control (otherwise the lower energy exo product would be the major product) and is instead under kinetic control.
From the endo product, it is considered what would happen if the molecule was hydrogenated. It is know that initial only one of the two carbon double bonds is hydrogenated first and that more forcing conditions are need to hydrogenate the second double bond. This gives rice to two possible products as shown in the reaction scheme below:
Using the MM2 force field calculation again, it is possible to find out which of the two possible products is lower in energy. As this reaction under thermodynamic control, which ever of the two possible products has the lower energy will be the major product of the reaction.