Rep:Module1:MaDfnif05
Modelling Using Molecular Mechanics
Hydrogenation of Cyclopentadiene Dimer


Cyclopentadiene dimerises in a Diels Alder cycloaddition. 6 π electrons(4n+2, n=1) are involved so thermally it proceeds with Huckel topology involving only suprafacial components. There are two modes of attack of the π4s and π2s components, endo and exo leading to two possible isomers, the exo dimer 1 and the endo dimer 2. The endo adduct is found to be the major product. Molecular mechanics MM has be used to optimise the geometries and calculate the energies of the two isomers,which can then be compared as the same force field MM2 is being used. The values are summarised in table 1. The exo isomer is lower in energy than the endo isomer. This implies that the reaction in kinetically controlled (irreversible conditions). If the reaction was under thermodynamic control (reversible conditions) then the more stable exo dimer would be expected to be the major product. The kinetic product is the one that forms fastest, that is the one with the lowest energy transition state. This implies that the transition state on route to the endo adduct is slightly more stable than that for the exo.
| Molecule | Total Energy kcalmol-1 |
|---|---|
| Exo 1 | 31.9 |
| Endo 2 | 34.0 |
| dihydro 3 | 35.7 |
| dihydro 4 | 31.2 |
The preference for the product of kinetic control is called Alder’s rule or the endo rule. The endo adduct is favoured due to secondary orbital interactions. There is a bonding interaction at the back of the diene. This interaction is not possible in the exo isomer.[1]. MM cannot deal with these type of interactions. It is a classical approach.Quantum mechanics is required. It doesn't consider the electronics. Bonds are put where told to go. MM uses data from experimentally known and characterised molecules and hence cannot be used to model kinetic reactions as it cannot paramatise the high energy transition states.
The endo dimer hydrogenates initially to one of the two dihydro derivatives 3 or 4. The total energies calculated for these molecules using the MM2 force field show that isomer 4 is lower in energy than 3. This total energy can be broken down into relative contributions from stretching(str), torsion (tor), Van der Waals (vdw) and hydrogen bonding terms (table 2). Stretches, bends and torsions indicate strain in molecule. The stretching energy terms are fairly similar in both isomers.
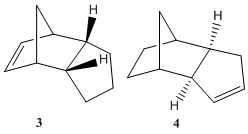
The bending energy is the largest contribution to the total energy in each case, implies that the angles are quite far from their natural values. This term is approximately 5 kcal/mol higher in derivative 3 and thus provides one explanation for the higher energy of 3 compared to 4. The torsion energy for 4 is however higher in energy than that for 3 although, this does not outweigh the higher bending energy of 3. (The reduction is bending energy of isomer 4 is accompanied by an increase in torsion energy). There are no atoms present to participate in hydrogen bonding however, the hydrogen atoms can interact. These may be incorporated in the 1,4 van der Waals energy term . This is higher in 3 than 4 (this energy would include the interaction across the ring between the hydrogen atoms specifically shown, which are four carbon atoms apart ). Hence 3 is likely to be higher in energy as a result of strain and destabilising hydrogen interactions.
On the basis of this result, isomer 4 would be expected to be the result of thermodynamic hydrogenation. However, if the hydrogenation mechanism involves a late stage transition state then by Hammond’s Postulate the transition leading to isomer 3 would be expected to be lower in energy and hence the activation energy barrier to forming 3 may be less than that to form 4. Therefore, isomer 3 could also be the product of kinetic control. Thermodynamic control can be achieved using metal catalysts.
| Energy Term | dihydro 3 | dihydro 4 |
|---|---|---|
| str | 1.27 | 1.10 |
| bnd | 19.8 | 14.6 |
| tor | 10.9 | 12.5 |
| 1,4 vdw | 5.64 | 4.50 |
Stereochemistry of Nucleophilic additions to a pyridinium ring(NAD+)
The NAD+ derivative 5 undergoes regioselective and stereoselective alkylation with the Grignard reagent to give 6. It reacts selectivity at the 4 position of the pyridinium ring and gives the diastereoisomer in which this Me group is anti to the hydrogen atom of the sterogenic centre marked with *. This was determined by X-ray crystallography by A. G. Schultz et al[2]. They proposed that the electropositive magnesium metal coordinates to a basic lone pair on the carbonyl oxygen of the amide.
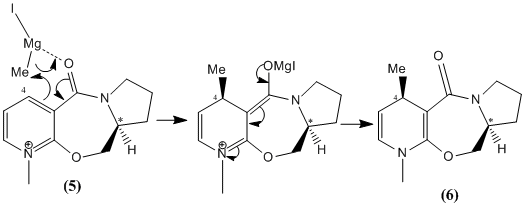
The methylation then takes place intramolecularly via a 6 membered transition state. Hence alkylation occurs at the 4 position. (can’t reach 2 position). The MM2 force field can be used to minimise the energy of 5 from several different starting points. Upon first optimisation, an energy of 43.1 kcalmol-1 was obtained and the dihedral angle (between the carbonyl oxygen and carbon 4 of the pyridinium ring) was measured at 12°.
The carbonyl was not quite coplanar with the ring but was above the plane such that it was trans to the hydrogen of the chiral centre. Attempts from different starting positions decreased the dihedral angle further towards coplanarity with little difference in energy. The smallest angle achieved was 8 degrees. A conformation in which the dihedral angle was negative, that is the carbonyl was below the plane, syn to the hydrogen of the chiral centre could not be achieved. The seven membered ring is not very flexible. Hence, the carbonyl group by coordinating to the Mg directs the methyl group (via an intramolecular reaction) to the same face of the pyridinium ring to which it is on, resulting in the stereochemistry shown.
The pyridinium ring in 7 undergoes a stereoselective nucleophilic addition with the amine, PhNH2 to give 8. Attack occurs at the 4 position.
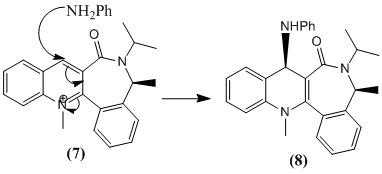
Optimization gave an intial energy of 63.4 kcal/mol and a dihedral angle of -19°. (The carbonyl group is 19° below the plane of the pyridinium ring , anti to the methyl group of the 7-membered ring.).The amine is a bulky nucleophile. It is also electron rich and hence will repel the carbonyl oxygen of the lactam. Thus on the basis of sterics and electrostatics the amine approaches the 4 position of the pyridinium ring from the opposite face to the carbonyl group, that is to end up cis to the lactam methyl group. The 7-membered ring is not very flexible.
The MeMgI component cannot be included because the program does not include the Mg atom type. MM2 atom types include for example, sp3 , sp2, sp carbon, N as well as the halogens. Metals are not included. The program doesn’t know want a Mg-C bond looks like.
The MM2 force field also warns that some parameters are being guessed at. Molecular mechanics adds together five non-interacting terms. The aromaticity of the pyridinium ring can’t be means the additive doesn’t apply as the bonds can’t be related to simpler diatomics.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
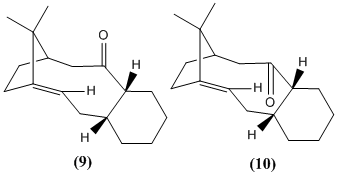
Either 9 (carbonyl group pointing up) or 10 (carbonyl group pointing down) is an intermediate in the synthesis of paclitaxel. Both isomers contain a bridgehead double bond. Bredt’s rule states that elimination towards a bridgehead is not observed. They are expected to be high in energy due to the strain of achieving a sp2 planar bridgehead carbon. Some of the strain could be relieved if ring was made larger. In very large rings, the double bond tends towards that of an acyclic. In certain ring sizes, these bonds can become very stable in which case they are called hyperstable olefins. Ref ok . Maier and Schleyer report that the strain is less than the parent hydrocarbon and that sterics hindrance and pi bond energy do not alone account for the unreactivity of such bonds.
The total energy of intermediates 9 and 10 found using MM2 force field are 48.9 and 44.3 kcal/mol, respectively. 10 is lower in energy than 9. The stereochemistry of the tetrahedral intermediate is thus determined by nucleophilic addition (at the Burgi-Dunitz angle) to the prochiral carbonyl of isomer 10.Cycloalkenes are usually more strained that their parent cycloaklanes. However, due to increased transannular hydrogen interactions in the cycloalkane here, the cycloalkene has a lower strain energy.[3] Hence this alkene reacted slowly during susquent reactions because its hyperstable, partly due to the size of the ring.[4] The bridgehead alkene is part of a 10 membered ring. Electrophiles may also not be able to approach the alkene so readily due to steric hindrance from the methyl group above and the carbonyl bond below.
The lowest energy of the structure seems to be achieved when the adjoining 6-membered ring is in the chair conformation.Of the three limiting conformations of cyclohexane, the chair and twist boat are energy minima whereas the boat is an energy maxima. The chair is lowest in energy (it allows all carbons to adopt sp3 tetrahedral geometry and minimised torisoal stain, all bonds are fully staggered) The total energy of the molecule was 54.1 kcal/mol when this ring was in the twist boat conformation.
10 |
9 |
Modelling Using Semi-empirical Molecular Orbital Theory
Regioselective Addition of dichlorocarbene
Orbital Control of Reactivity
 |
 |
 |
 |
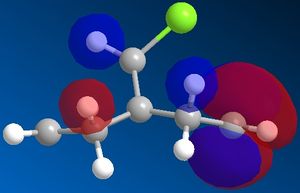 |
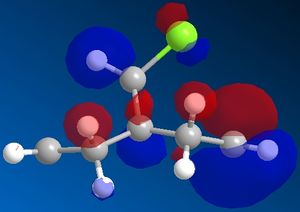
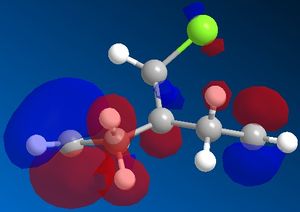
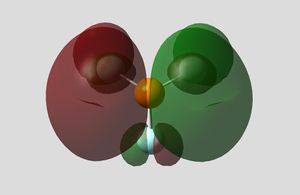
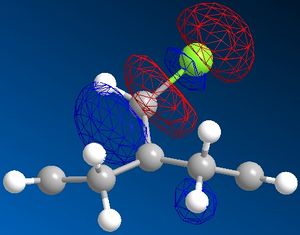
Pre-optimisation of the geometry of 12 using the MM2 force field followed by a quantum mechanical treatment; MOPAC/PM6 method generates an approximate representation of the valence-electron molecular wavefunctions. The HOMO, HOMO-1, LUMO, LUMO+1 and LUMO+2 are shown. This method discriminates between the two alkenes. The HOMO indicates that the alkene endo to the chloro group (underneath the chlorine) is the most nucleophilic as the PM6 calulation of this orbital has a larger coefficient at this alkene compared to the one anti to the Cl group.This agrees with experiment [5] which shows that electrophilic addition occurs regioselectivity at this alkene double bond. Attack is also found to take from the face opposite the bridge presumably because of sterics. The explanation given [6]is that there is an interaction between the exo (anti) pi bonding orbital and the carbon of the C-Cl sigma antibonding orbital (a so called negative sigma conjugation or hyperconjugation). These two orbitals are in an antiperiplanar arrangement (app). The C-Cl sigma antibonding orbital is represented by the LUMO+1 and the pi bonding orbital of the exo alkene by HOMO-1. The phases are clearly correct for orbital overlap(overall integral). This stabilising interaction effectively removes some electron density from the exo pi orbital (and into the sigma orbital of the C-Cl bond) lowering its energy relative to endo. (HOMO-1 more delocalised). It is also predicted that the anti (or exo) double bond will distort towards the bridgehead due this stereoelectronic effect. In fact when the PM6 calculation is run this distortion can be observed.
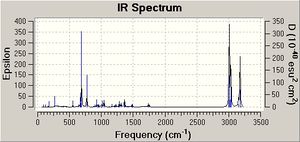
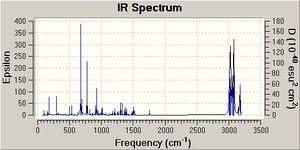
Influence of C-Cl bond on vibrational frequencies
Comparison of the vibrational frequencies of compound 12 (specifically the C-Cl and C=C stretching modes) with those of the monohydrogenation derivative 13 in a way provide further evidence for the interaction. Both geometries can be optimised to the B3LYP/6-31G(d,p) level and the vibrational frequencies calculated. The 2 C=C stretching frequencies in 12 are seen at 1737 and 1757 wavenumbers for the exo and endo alkenes, respectively. Clearly, the two stretching frequies are different. Donation of electron density from the pi to sigma antibonding weakens the exo double bond relative to the endo and so it appears at lower wavenumbers (wavenumber is proportional to energy). Hydrogenation of this exo double bond to give the monoene, shows the endo C=C stretch at 1758 wavenumbers. C-Cl stretching frequeincs are also observed at 771 and 775 cm-1 for 12 and 13 , respectively.
Mini Project
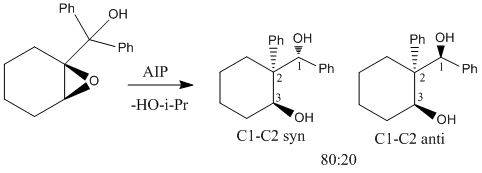
Tu et al [7] report the stereoselective reductive rearrangement of several epoxides with aluminium isopropoxide (AIP), namely that of 2-(1-hydroxy-1-phenylcyclohexanol) to give the 1,3-diol product as a mixture of C1 diastereoisomers.The Lewis acidic AIP reacts with the alpha hydroxy group of the epoxide. The resulting intermediate then undergoes a semi-pinacol rearrangement followed by a Meerwein-Pondorf Verley type reduction. The resulting products each contain three stereoenic centres. Only the stereochemistry of the hydroxyl group at carbon 1 differs between the two isomers. Tu et al found and 80:20 mixture of the diastereoisomer with the phenyl on carbon 2 and the hydroxyl at position 1 in the syn arrangement relative to the C1-C2 anti isomer. They identified the stereochemistry of the products using a technique of 1H-1H-NOESY. Irradiation of the hydrogen at C1 would be expected to show a strong NOE (large signal enhancement) to the hydrogen at C3 in the minor (anti) isomer indicating that they are on the same of the molecule. The NOE to the same hydrogen in the major isomer would be expected to be weaker as they are further apart[8]. The geometries of both stereoisomers were fully optimised at the mpw1pw91/6-31(d,p) level. Rotation about the C1-C2 single bond could give rise to several conformations. However, the preferred conformation places the two phenyl groups as far apart as possible presumably on the grounds of sterics. The preferred conformation for the cyclohexane ring is the chair (as above). The 13 C NMR cehimcal shifts were calculated (table 3) using the GIAO approach. This uses quantum mechanical density functional theory. One drawback is that it is conformational sensitive.
| Major | ||||||||||
| Calculated | 23.3 | 25.8 | 32.1 | 38.8 | 53.1 | 73.8 | 74.3 | 121.3-128.3 | 140.8 | 142.0 |
| Literature | 18.9 | 19.9 | 20.7 | 29.4 | 49. 3 | 74.0 | 82.9 | 125.9-128.5 | 140 | 140.5 |
| Assignment | 1 | 2 | 3 | 6 | 5 | 14 | 4 | aromatic | 15 | 8 |
| Minor | ||||||||||
| Calculated | 25.6 | 26.4 | 34.5 | 42.9 | 51.7 | 76.7 | 84.6 | 121.6- 125.8 | 140.4 | 144.9 |
| Literature | 19.6 | 21.2 | 26. 7 | 28.9 | 49.5 | 67.0 | 81.4 | 125.9- 128.5 | 139.8 | 140.4 |
| Assignment | 1 | 2 | 3 | 6 | 5 | 4 | 14 | aromatic | 15 | 8 |
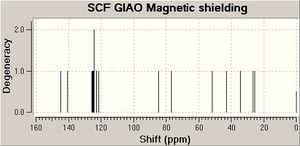
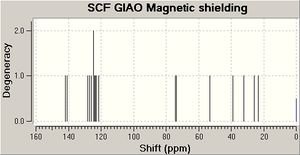
In general, there is good agreement between the experimental literature values and those calculated. It is possible to distinguish the two diasteroisomers from the calculated shifts. The 1H NMR calculations are less accurate, so have not be used.3J coupling constant calculations will probably not in this case aid in assigning the structures of the isomers as the all 3J couplings present are in the aromatic phenyl rings.
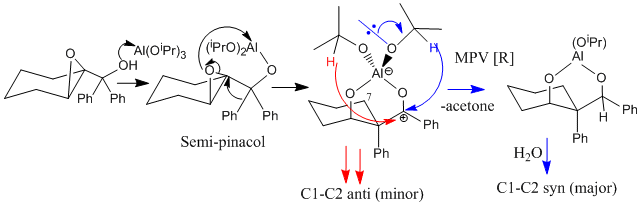
The stereoselectivity of the reaction can be explained by considering the mechanism. The stereoselectivity of the semi-pinacol rearrangement is goverened by stereoelectronics (calculated MOs may be useful in showing this). Coordination of the epoxide oxygen to the lewis acid faciliates the ring opening. This is SN1 like and hence the developing positive charge will occur at the more substituted quarternary carbon. The C(2)-O bond will break. One of the phenyl groups then undergoes a 1,2- C shift towards the developing charge. Effectively,σ (CH) to p(vac) antiperiplanar (app) interaction. As both alkyl groups are phenyls there is no issue of migration preference. Migration aptitude would be expected to follow the same order of carbocation stabilising abilty. The resulting aluminium tertiary carbocation undergoes a Meerwein-Pondorf Verley type reduction giving acetone as a by-product. The Al atom is tetrahedral. Two possible hydride shifts to the planar carbocation centre can occur (red and blue). The red hydride of the isopropoxy group is facing backwards over the cyclohexane ring. Hence there is an interaction with the axial hydrogen on carbon 7. This is not the case this the blue hydride so this is the major pathway.
References
- ↑ J. Clayden, N. Greeves, S. Warren, and P. Wothers, Organic Chemistry, OUP, 2007, pp. 917
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
- ↑ P.Camps, X. Pujol, S.Vazquez, M.A. Pericas, C.Puigjaner and L.Sola, Tetra.,2001, 57,8511DOI:10.1016/S0040-4020(01)00802-X
- ↑ W.F Maier and R. Schleyer, J.Am.Chem.Soc.,103,1981
- ↑ B. Halton and S.G.G Russell, J. Org. Chemistry, 1991, 56, 5553. DOI:10.1021/jo00019a015
- ↑ B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P29920000447
- ↑ Y.Q Tu, L.D.Sun and P.Z Wang,J. Org. Chem,1996,64, 629 DOI:10.1021/jo981097z
- ↑ Rob Law's NMR spectroscopy notes,Yr 2
