Rep:Module1:JYX08
Part 1: The basic techniques of molecular mechanics and semi-empirical molecular orbital methods for structural and spectroscopic evaluations
Introduction
Many organic reactions can give rise to multiple isomeric products. It is often difficult to predict which one would predominate. However it is possible to use various computer programs to predict the most thermodynamically stable conformation of the product using molecular modelling. This calculated result can then be used to explain some of the reactivity and selectivity in certain reactions, although other factors will also needed to be taken into consideration as this is purely based on mathematical calculation. In this exercise, Allinger's MM2[1] force field employed by ChemBio3D Ultra 12.0 will be used to investigate few reactions hence predicting the resulting product.
Hydrogentation of Cyclopentadiene Dimer
Dimerisation of Cyclopentadien
Wilson[2] and co-workers have reported that Cyclopentadienes can rapidly dimerise at room temperature via Diels-Alder[3]reaction to give one of the possible products, exo 1 and endo 2. The reaction mechanism is shown below:
Since either form is possible, we would then compare their total energies using MM2 force field to determine which one is the most stabilised product, as shown in the table blow:
| Dimer | Energy / kcal mol-1 | Energy / kJ mol-1 | |||
exo-dicyclopentadiene 1
|
31.88 | 133.46 | |||
endo-dicyclopentadiene 2
|
34.01 | 142.34 |
From the calculated results above, we can clearly see that the exo product would be the more favourable as it has the lowest energy, thus more thermodynamically stable compare to the endo product. This of course would be the expected outcome if the reaction was run under thermodynamic conditions, and this was indeed proven by Herndon and his co-workers. However at room temperature, the reaction is under kinetic control, which means that the most stable transition state will lead to the major product. According to the endo rule[4], a 'maximum accumulation of double bonds' will lead to the most stable transition state, thus a more rapid overcome of the transition state barrier. This observation can also be considered by the "secondary orbital" overlapping interactions for pericyclic reactions.
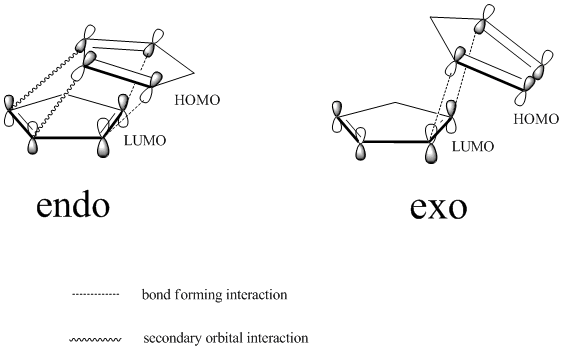
Therefore since formation of endo product is fast and has a more stabilised transition state, we can conclude that the endo product is the actual dominating species.
Hydrogenation of Cyclopentadiene Dimer
Hydrogenation of the endo-isomer can potentially give rise to two isomers 3 and 4[5]. Further hydrogenation after prolonged hours can result in complete hydrogenation of both of the double bonds.
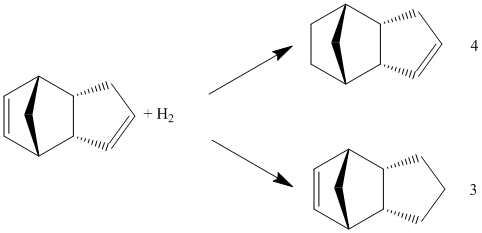
The energies of the possible dihydro products were again calculated using MM2. Breakdown of the total energies for each isomer were recorded in a table below for comparison.
| Contributions of Energy | Dihydro derivative 3 | Dihydro derivative 4 | |||||||
|
| ||||||||
| Energy/kcalmol-1 | Energy/kJmol-1 | Energy/kcalmol-1 | Energy/kJmol-1 | ||||||
| Stretching (Str) | 1.3 | 5.3 | 1.1 | 4.6 | |||||
| Bending (Bnd) | 19.9 | 83.1 | 14.5 | 60.8 | |||||
| Torsion (Tor) | 10.8 | 45.3 | 12.5 | 52.3 | |||||
| Van der Waals(VdW) | 5.6 | 23.6 | 4.5 | 18.9 | |||||
| Hydrogen Bonding (H-Bond) | 0.2 | 0.7 | 0.1 | 0.6 | |||||
| Total Energy | 35.7 | 149.4 | 31.2 | 130.4 |
From the table above, we can clearly see the large difference in bending energies of isomer 3 and 4. This can be attributed to the higher steric strain experienced by norbornene C=C bond in conparison to the cyclopentene C=C bond. The relief of ring strain through hydrogenation of the C=C bond in the norbornene unit will be favoured over the hydrogenation of the cylopentene part. Thus for isomer 3 the ring stain inside the norbornene still remains, hence it is the unfavourable product.
Thus, the major dihydro derivative of endo-cyclopentadiene is product 4. This prediction is supported by actual experimental data, which showed that the rate of hydrogenation of the double bond in the norbornene ring to form the dihydro derivative 4 is many times higher than hydrogenation of the cyclopentene ring to give dihydro derivative 3[6].
Stereochemistry of Nucleophilic Additions to a Pyridinium Ring (NAD+ analogue)
Reaction of Pyridinium Ring Prolinol derivative 5 with Grignard Reagent
The nucleophilic attack of the Grignard reagent[7] on is very stereo- and regio- selective[8]. The overall reaction scheme is shown below. It involves the grignard attack on the 4 position of the pyridine ring, which results in the methyl group being added at the 4 position with the stereochemistry shown.This reaction has also been previously studied in the literature.
This absolute stereo selective mechanism shown on the right can be credited to the chelation of the carbonyl oxygen onto the Mg. The reason for methyl group specifically adds onto the top phase of the hetero aromatic ring was investigated using MM2.
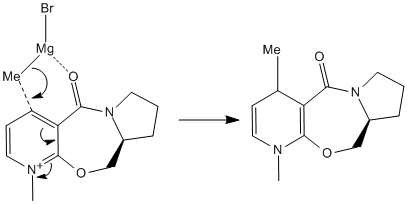
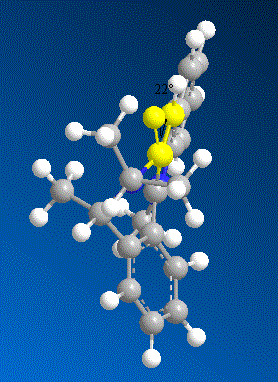
The table below shows the total energies of the molecule 5 while changing the dihedral angle which is created by the carbonyl oxygen and the carbon on the aromatic ring. The computer simulated models showed that the carbonyl group was infact coming out of the plane instead of sitting in the plane of the molecule. Further more, the molecule is in its most stabilised form when the dihedral angle is 11°. As previously mentioned that the mechanism involves a chelation of O to Mg which will keep the Grignard reagent fixed in place, thus the methyl group will always be delivered onto the ring on to the same phase.
| Contributions of Energy | Conformer a | Conformer b | Conformer c | Conformer d | |
| Energy/kcalmol-1 | Energy/kcalmol-1 | Energy/kcalmol-1 | Energy/kcalmol-1 | ||
| Stretching (Str) | 3.88 | 2.03 | 2.05 | 2.06 | |
| Bending (Bnd) | 115.55 | 14.21 | 14.27 | 14.26 | |
| Torsion (Tor) | 33.56 | 5.14 | 5.06 | 5.03 | |
| Van der Waals(VdW) | 25.44 | 16.54 | 16.54 | 16.57 | |
| dihedral angle | +122° | +11° | +10° | +9° | |
| Total Energy | 168.21 | 43.12 | 43.11 | 43.12 |
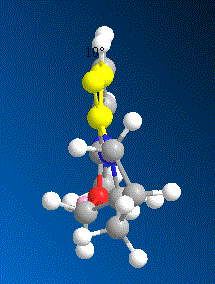
Nucleophilic Addition of Aniline to the N-methyl quinolinium Ring
Similarly, nucleophilic addition of aniline to N-methyl quinolinium salt 7 [9] is highly regio- and stereo- selective.In the product (8), similar to the previously discussed Grignard reagent addition reaction, that the nucleopile of the aniline group is added specifically to one face the pyridinium ring. However the main difference in this reaction is that the orientation of the carbonyl group is on the opposite face of the attack.
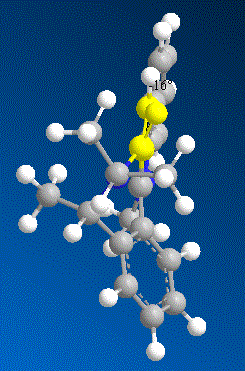
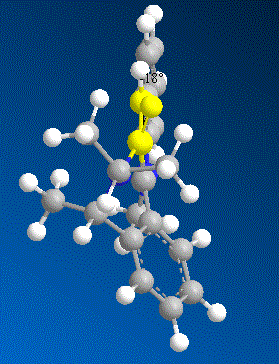
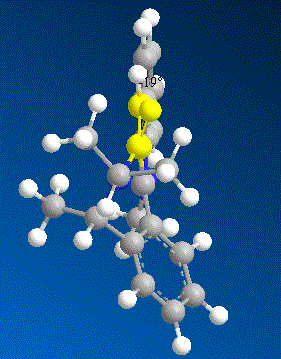
In order to understand why this reaction is again a stereo selective reaction, several models of molecule 7 were made with varying dihedral angles between the carbonyl and the C4 position on the pyridinium ring in ChemBio 3D and the total energy was minimised using MM2 Force Field.
| Contributions of Energy | Conformer a | Conformer b | Conformer c | Conformer d | |
| Energy/kcalmol-1 | Energy/kcalmol-1 | Energy/kcalmol-1 | Energy/kcalmol-1 | ||
| dihedral angle | +22.6° | -16.8° | -18° | -19° | |
| Total Energy | 84.17 | 63.74 | 63.55 | 64.27 |
From the results shown above, it is clear that the conformer D is the most thermodynamically stable conformer with dihedral angle of -19°. The orientation of the carbonyl group results from the steric strain of the three bulky methyl groups which are all on the top-face of the molecule and hence “push” the Oxygen out of plane. On the other hand, the nucleophile of aniline does not coordinate to the carbonyl group to undergo an intramolecular addition as the Grignard reagent. In fact the lone pair on the nitrogen and the very electron rich phenyl group will result in strong repulsion to the lone pairs on the oxygen of the carbonyl group. Therefore the nucleopile would prefer to approach to the attack site from the opposite face to the carbonyl group.
Possible Improvements
Alternatively we could use other more powerful and accurate computer programs such as Dewars’ MOPAC method and Poples’s Gaussian density functional theory (DFT) method instead of MM2 molecular mechanics force field. Although MOPAC and DFT requires are more computationally intensive and requires very powerful machines to carry out the calculation, they give rather accurate results by taking into the account of the electronic structures of the molecules, and are thus give better calculations of the energy and geometry optimisation. Moreover, Gaussian is also able to simulate two molecules with respect to each other in the same calculation, and thus gives a better view of the reaction profile, hence a better explanation of the regio- and stereo- issues in a reaction. ChemBio 3D on the other hand is not so great for such reaction is not only because it is not powerful enough, it is unable to recognise the Grignard reagent coordinating to the carbonyl oxygen. The other problem rose in the reaction of molecule 7 and the aniline, as it is unable to optimise the energy of the two molecules with respect to each other, only individually, and therefore this does not give a good approximation of the geometry of the molecule being attacked when the nucleophile approaches.
Introducing bulky substituents to the alkyl ring would also help to restrain the conformationally of the ring. This creates larger activation energy for the ring “flipping” mechanism, and makes it unfavorable to “flip”. Alternatively ring-locking groups can also benefit the computational calculations when there is fewer possibilities for the optimised conformation of the molecule.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
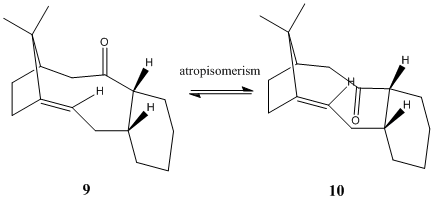
Atropisomerism of 9 and 10
atropisomer are stereoisomers in which steric strain hinders rotation about single bonds and prevents interconversion of isomers.
and are important intermediates in the synthesis of the anti-cancer drog Taxol.
The energies of the atropisomers were calculated using MM2, and compared, as shown in the table below.
| Atropisomers | Stretching / kJ mol-1 | Bending / kJ mol-1 | Torsion / kJ mol-1 | Van der Waals / kJ mol-1 | Hydrogen Bonding / kJ mol-1 | Total Energy / kJ mol-1 |
|---|---|---|---|---|---|---|
| Intermediate 9 | 11.6 | 67.2 | 87.7 | 57.4 | 0.5 | 223.04 |
| Intermediate 10 | 10.5 | 44.2 | 80.9 | 51.5 | -0.74 | 181.7 |
Atropisomer 9 has the geometry of a twisted boad like conformation with the carbonyl pointing up, this results in a greater bending and torsion strain as the bulky ring is forced to bend towards eachother. 10 on the other hand adopts a slightly flatter chair conformation with the carbonyl pointing down. This has significantly reduced the tortion energy by about 23 kJ mol-1, and as well as the bending energy. Therefore overall 10 is a more thermodynamically stable intermediate. MMFF94 Energies were also calculated for each atropisomer, and it gave a similar but slightly higher set of results, which again indicates that 10 being in a lower energy than 9.
Hyperstability[10] of 9 and 10
This intermediate contains an alkene bond next to a bridging methylene unit. If we would try to react with this double bond under normal conditions, the result will be very poor, and in fact nothing will happen due to the so called "hyperstability" of olefins. The definition of "hyperstable olefins" defined by Maier[11] and Schleyer are alkenes which are less strained than their parent saturated hydrocarbons. This was originally discovered by Wiseman as he has discovered the high stability of bridgehead olefins contained in a cylcoalkene. The reason for such compound being so stable is due to the effect known as negative olefin strain. The C next to the bridge is in a sp2 geometry, this makes it suffer less from the steric hinderance at the bridge. If this product was successfully hydrogenated, this will result in a higher ethalpy than the starting molecule as the hydrogenated species will suffer more from the steric strain.
Structure based Mini project using DFT-based Molecular orbital methods
Semmelhack[12] and co-workers has invented a way using Pd catalyst to synthesis fused-γ-pyran lactone. Semmelhack's method has been recognised as one of the most valuable synthetic method. This reaction apparantly is stereo- selective as it only forms one of the stereo-isomers. In the product, the two hydrogen atoms can either be trans or cis to each other (as shown in the blow reaction scheme). In this study the experimentally obtained 13C NMR spectra will be compared to calculated spectra. The reaction producing the two isomers is shown below:
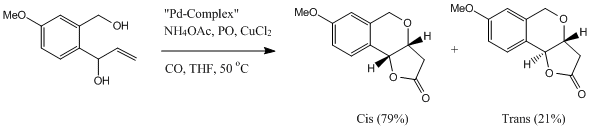
| Contributions of Energy | cis-isomer | ||||
|
|||||
| Energy/kcalmol-1 | Energy/kJmol-1 | ||||
| Stretching (Str) | 1.1 | 4.51 | |||
| Bending (Bnd) | 5.9 | 24.19 | |||
| Torsion (Tor) | -9.2 | -37.7 | |||
| Van der Waals(VdW) | 15.0 | 61.5 | |||
| Dipole/Dipole | 6.9 | 28.3 | |||
| Total Energy | 17.6 | 72.16 |
| Contributions of Energy | trans-isomer | ||||
|
|||||
| Energy/kcalmol-1 | Energy/kJmol-1 | ||||
| Stretching (Str) | 2.3 | 9.4 | |||
| Bending (Bnd) | 60.1 | 246.4 | |||
| Torsion (Tor) | -8.4 | -34.4 | |||
| Van der Waals(VdW) | 18.6 | 76.3 | |||
| Dipole/Dipole | 4.3 | 94.7 | |||
| Total Energy | 23.1 | 94.7 |
From the above calculation done by the MM2, we can see that the total energy for the cis isomer is lower than the trans, which means that cis isomer is more thermodynamically favoured product. This corresponds to what is observed in the literature.[13]
A comparison between the experimentally otained and predicted NMR spectra will now be made.
Cis-isomer 13C NMR spectra
The NMR spectra for the cis-isomer can be seen below. Please click on the images to enlarge them.
-
Experimentally obtained 13C NMR of cis-isomer[14]
-
Predicted 13C NMR of cis-isomer
| Carbon atom | Lit. value/ppm | Calculated value/ppm | difference/ppm |
|---|---|---|---|
| 15 | 37.7 | 38.6 | 0.9 |
| 10 | 55.3 | 53.5 | 1.8 |
| 8 | 66.8 | 66.7 | 0.1 |
| 12 | 73.3 | 73.7 | 0.4 |
| 9 | 75.5 | 74.6 | 0.9 |
| 2 | 108.9 | 106.1 | 2.8 |
| 6 | 113.8 | 109.7 | 4.1 |
| 4 | 119.7 | 117.9 | 1.8 |
| 3 | 132.0 | 130.0 | 2 |
| 5 | 136.6 | 134.8 | 1.8 |
| 1 | 160.1 | 154.9 | 5.2 |
| 14 | 174.9 | 169.4 | 5.5 |
The average difference in ppm is 2.19 ppm. This therefore shows that the calculated NMR data compares well to the literature values.
Trans-isomer 13C NMR spectra
The NMR spectra for the trans-isomer can seen below. Please click on the images to enlarge them.
-
Experimentally obtained 13C NMR of trans-isomer[15]
-
Predicted 13C NMR of trans-isomer
| Carbon atom | Lit. value/ppm | Calculated value/ppm | difference/ppm |
|---|---|---|---|
| 15 | 36.0 | 37.1 | 1.1 |
| 10 | 55.3 | 53.5 | 1.8 |
| 8 | 69.6 | 69.1 | 0.5 |
| 12 | 76.3 | 75.9 | 0.4 |
| 9 | 77.9 | 76.9 | 1 |
| 2 | 109.9 | 105.7 | 4.2 |
| 6 | 112.4 | 110.4 | 2 |
| 3 | 124.8 | 123 | 1.8 |
| 4 | 125.3 | 123.0 | 2.3 |
| 5 | 134.4 | 132.8 | 1.6 |
| 1 | 159.4 | 154.0 | 5.4 |
| 14 | 172.9 | 168.0 | 4.9 |
The average difference in ppm is 2.25 ppm. This therefore shows that the calculated NMR data compares well to the literature values.
Both of the predicted 13C NMR spectra showed very good agreement with the experimental observations, this shows that Gaussian DFT is a liable method.
Discerning Isomers via GIAO 13C NMR Predictions
The calculated 13C NMR can easily distinguish between the cis and trans isomers, especially:
The ordering in chemical shift values for C-3 and C-4 has reversed for the cis and trans isomer, which corresponds to the "flip" of the C-H bonds from cis conformation to trans.
C-3 has chemical shift δ = 124.8 ppm for trans and δ = 132 ppm for cis isomer.
C-4 has chemical shift δ = 125 ppm for trans and δ = 119.7 ppm for cis isomer.
Conclusion
This method has successfully differenciated the two isomers, and the chemical shifts in the calculated 13C NMR spectra agrees to the experimental values shows that this method worked very well. The MM2 method has successfully calculated the energies of the molecules and optimised the geometry of the isomers. The calculated energies also corresponds to the experimental observation, which prooves that the paper was correct.
References
- ↑ N.L.J. Allinger, CJ. Am. Chem. Soc., 1977, 99, 8127: DOI:10.1021/ja00467a001
- ↑ P.J. Jr. Wilson, J.H. Wells, Chem. Rev., 1944, 34, 1: DOI:10.1021/cr60107a001
- ↑ J. Sauer, R. Sustmann, Angew. Chem, 1980, 19, 779: DOI:10.1002/anie.198007791
- ↑ K. Alder, G. Stein, Angew. Chem, 1937, 50, 514: DOI:10.1002/ange.19370502804
- ↑ D. Skála, J. Hanika, Pet. Coal, 2003, 45, 105: PDF
- ↑ Kirk-Othmer: Encyclopedia of Chemical Technology, 2nd Ed., Vol 6, p.688, John Wiley and Sons, 1985
- ↑ Y.H. Lai, Synthesis, 1981, 1981, 585: DOI:10.1055/s-1981-29537
- ↑ A.G. Shultz, L. Flood, J.P. Springer, J. Org. Chem., 1986, 51, 838: DOI:10.1021/jo00356a016
- ↑ S. Leleu, C. Papamicaël, F. Marsais, G. Dupas, V. Leavacher, Tetrahedron: Asymmetry, 2004, 15, 3919: DOI:10.1016/j.tetasy.2004.11.004
- ↑ W.F. Maier, P.v.R. Schleyer, J. Am. Chem. Soc., 1981, 103, 1891: DOI:10.1021/ja00398a003
- ↑ 'Evaluation and prediction of the stability of bridgehead olefins' - Wilhelm F. Maier, Paul Von Rague Schleyer -Journal of the American Chemical Society 1981 103 (8), 1891-1900
- ↑ Semmelhack, M. F.; Zask, A. J. Am. Chem. Soc. 1983, 105, 2034DOI:10.1021/jo00280a003 10.1021/jo00280a003
- ↑ Diversity-Oriented Synthesis of Fused Pyran γ-Lactones via an Efficient Pd−Thiourea-Catalyzed Alkoxycarbonylative Annulation,Zhengtao Li, Yingxiang Gao, Zhaodong Jiao, Na Wu, David Zhigang Wang, Zhen Yang, Organic Letters 2008 10 (22),5163-5166.DOI:10.1021/ol802115u
- ↑ Diversity-Oriented Synthesis of Fused Pyran γ-Lactones via an Efficient Pd−Thiourea-Catalyzed Alkoxycarbonylative Annulation,Zhengtao Li, Yingxiang Gao, Zhaodong Jiao, Na Wu, David Zhigang Wang, Zhen Yang, Organic Letters 2008 10 (22),5163-5166.DOI:10.1021/ol802115u
- ↑ Diversity-Oriented Synthesis of Fused Pyran γ-Lactones via an Efficient Pd−Thiourea-Catalyzed Alkoxycarbonylative Annulation,Zhengtao Li, Yingxiang Gao, Zhaodong Jiao, Na Wu, David Zhigang Wang, Zhen Yang, Organic Letters 2008 10 (22),5163-5166.DOI:10.1021/Ol802115U




![Experimentally obtained 13C NMR of cis-isomer[14]](/images/thumb/d/dd/13C_NMR_cis_exp.jpg/120px-13C_NMR_cis_exp.jpg)

![Experimentally obtained 13C NMR of trans-isomer[15]](/images/thumb/4/4f/13C_NMR_trans_exp.jpg/120px-13C_NMR_trans_exp.jpg)
