Rep:Module1:Dima1407
Cyclopentadiene dimer
| Exo dimer (1) | Endo dimer (2) |
|---|---|
 |
 |
Exo dimer:
Stretch: 1.3207 Bend: 20.5691 Stretch-Bend: -0.8445 Torsion: 7.6812 Non-1,4 VDW: -1.4263 1,4 VDW: 4.2193 Dipole/Dipole: 0.3780 Total Energy: 31.8975 kcal/mol
Endo dimer:
Stretch: 1.2511 Bend: 20.8553 Stretch-Bend: -0.8319 Torsion: 9.5073 Non-1,4 VDW: -1.5238 1,4 VDW: 4.3003 Dipole/Dipole: 0.4460 Total Energy: 34.0043 kcal/mol
Exo product is more stable, than Endo. But because product of dimerisation reaction is Endo we can say that reaction is kinetically controlled.
Dihydro derivatives Molecule 3:
Stretch: 1.2812 Bend: 19.7597 Stretch-Bend: -0.8346 Torsion: 10.8768 Non-1,4 VDW: -1.1852 1,4 VDW: 5.6296 Dipole/Dipole: 0.1621 Total Energy: 35.6895 kcal/mol
Molecule 4:
Stretch: 1.0999 Bend: 14.5421 Stretch-Bend: -0.5462 Torsion: 12.5079 Non-1,4 VDW: -1.0727 1,4 VDW: 4.5003 Dipole/Dipole: 0.1407 Total Energy: 31.1720 kcal/mol
As we can see significant energy difference is due to bend component - difference in bend is 5.22 kcal/mol. C-C=C angle is 107.6(by dehydration of this double bond in Endo dimer gives us molecule 4). C-C=C angle is 112.8(by dehydration of this double bond in Endo dimer gives us molecule 3). So if Endo dimer will be dehydrated to give molecule 4, the angle will be close to tetrahedral, which is far more energetically effective.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue).
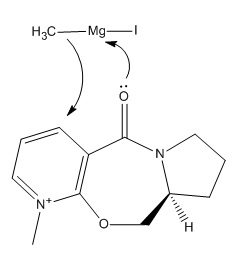
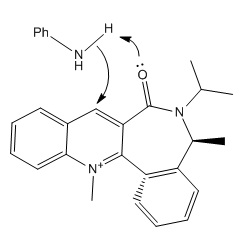
Grignard reagent reacts with Compound 5 highly regioselective, CH3 group attaches at the same side with respect to carbonyl oxygen[1]. The structure of optimised Compound 5 is shown in first jmole, with minimum energy 93.91020 Kcal/Mol.
Minimising energy/geometry using MOPAC (minimum is 155.47360 Kcal/Mol) we get the structure of the Compound 7 (second jmol). The reaction with aniline occurs on the opposite face with respect to C=O which is in accord with literature[2]
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol.

Using MM2:
Total Energy Compound 9: 54.1063 kcal/mol ( Bend: 19.0334)
Total Energy Compound 10: 44.9778 kcal/mol ( Bend: 12.8618)
Using MMFF94:
Total Energy Compound 9: 76.4217 kcal/mol
Total Energy Compound 10: 61.0542 kcal/mol
Comparing energies for Compound 9 and 10 received from MM2 minimizing energy we can say that Compound 10 is more stable. There are 2 probable reasons: repulsion between CH3 group (marked as 1 on the picture) and oxygen atom; due to the steric reasons they are located very close to each other and because of bend difference, which appears due to angles of the carbonyl group in Compounds 9 and 10 are 115.3, 118.2, 126.2 and 119.2, 120.2, 120.5 respectively.
Analysing MMFF94 we have the same result - Compound 10 is more stable.
Compounds 9 and 10 are hyperstable due to bridging C2H4 grop on te C=C bond in the cycle[3]. If we will hydrogenate Compound 10 we will get very unstable compound with highly unfavourable bond angles:
C7: 100.6, 104.4, 107.3, 109.0, 111.5, 123.6
C9: 101.3, 103.5, 104.1, 109.1, 111.5, 124.2
while in compound 10:
C7: 107.1, 119.7, 132.0
C9: 113.6, 116.3, 129.5
In case when atoms with the bigger size then H are bonded to C=C, the angles are even worse.
Modelling Using Semi-empirical Molecular Orbital Theory.
| Molecular orbitals for Compound 12 | ||||
|---|---|---|---|---|
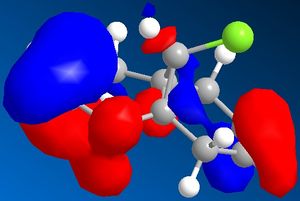 |
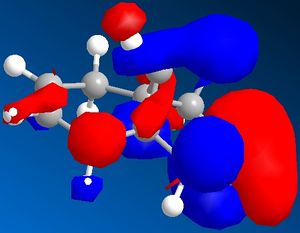 |
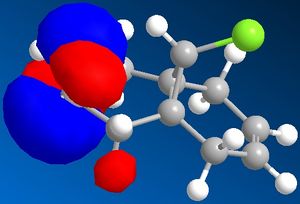 |
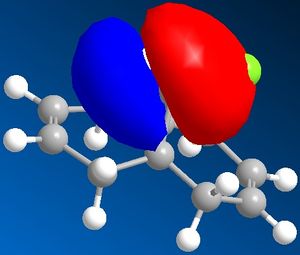 |
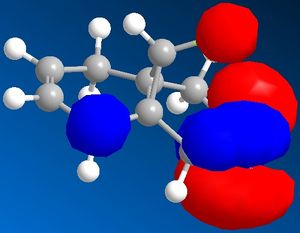 |
| Molecular orbitals for Compound 12 hydrogenated | ||||
|---|---|---|---|---|
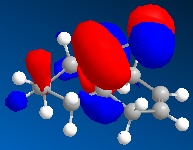 |
 |
 |
 |
 |
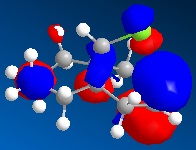
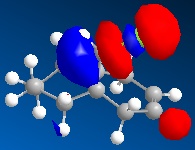
As we can see carbon double bond are very different due to orientation of chlorine atom. Carbon double bond which is at the same side with chlorine atom is more likely to react with electrophilic reagents such as for example dichlorocarbene, due to higher electron density in HOMO (better nucleophile) which is in accord with the literature[4].
In hydrogenated spices MOs have changed due to different symmetry caused by structural modifications.
| Bond | Compound 12 (freq./cm-1) | Compound 12 Intensity | Compound 12 hydrogenated (freq./cm-1) | Compound 12 hydrogenated Intensity |
|---|---|---|---|---|
| Cl-C | 771 | 25.13 | 775 | 20.01 |
| C=C | 1756 | 3.94 | 1758 | 4.34 |
| C=C anti to the Cl-C | 1737 | 4.20 | - | - |
As we can see from the table, only small changes occur in the peaks frequency. Frequency has increased - bond length has decreased, so carbon double bond has become more stable and unreactive, which is in accord with our MO for molecule with exo-hydrogenated bond (HOMO). A bit bigger change in intensities.
Mini-project.
Determination of the isomeric structure is a very important task In problems related to natural product chemistry, medicinal chemistry, etc. X-ray diffraction analysis is limited due to absence of suitable crystals; IR spectroscopy gives us information about group presence and not their sequence; chemical methods are sometimes too complicated (sometimes impossible). So the best way to use NMR spectroscopy.
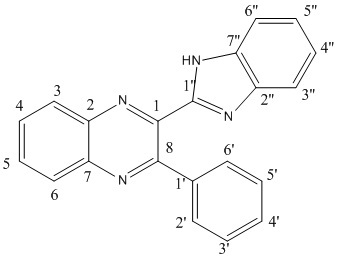
Compounds A and B are synthesised by the condensation reaction of 3-benzoylquinoxalin-2-one with 4-nitro-1,2-phenylenediamine, by the scheme[5]:
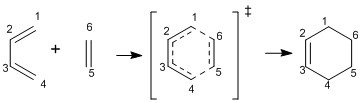
As there is no steric reasons not to form any of the isomer, I would expect that equal amounts of A and B would be produced.
The difference in 2 geometric isomers is location of the nitro group. So we should observe difference in benzene ring at which this group is attached. What we would expect is a big difference in peaks C5 and C4 due to high electron withdrawing NO2 group - higher shift at C4 is expected in the compound A and at C5 in the compound B.
To decide which product is formed in the reaction comparing real NMR spectrum[6] and predicted one could be very useful:
| Isomer | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1' | 2' | 3' | 4' | 5' | 6' | 1" | 2" | 3" | 4" | 5" | 6" | 7" |
| A literature | 147.12 | 140.12 | 126.13 | 149.56 | 127.77 | 132.39 | 144.87 | 157.65 | 139.47 | 131.04 | 129.17 | 131.04 | 129.17 | 131.04 | 149.98 | - | - | 124.66 | 124.66 | - | - |
| A calculated | 148.19 | 142.46 | 124.95 | 151.34 | 129.20 | 129.28 | 148.19 | 161.79 | 135.24 | 127.94 | 125.25 | 128.09 | 123.17 | 130.02 | 151.63 | - | - | 119.89 | 123.31 | - | - |
| B literature | 147.56 | 143.78 | 132.10 | 125.35 | 149.86 | 126.41 | 141.33 | 156.97 | 139.48 | 130.98 | 129.17 | 130.84 | 129.17 | 130.98 | 149.97 | - | - | 123.92 | 123.92 | - | - |
| B calculated | 147.78 | 145.66 | 127.64 | 122.12 | 150.96 | 125.25 | 143.54 | 160.5 | 141.06 | 127.04 | 124.95 | 127.04 | 122.57 | 128.98 | 151.34 | - | - | 119.89 | 123.31 | - | - |
"-" - are not observed due to extensive broadening and due to this have not been calculated.
As we can see from the Table reported literature values are in the good accordance (between 1 and 4.5 ppm difference) with the values calculated using GIAO method. According to calculated chemical shifts of the benzene fragment of the quinoxaline should be noticeably different for the isomers A and B. For example, C2 and C7 should resonate at δ 142.46 and 148.19 ppm in A versus 145.66 and 143.54 ppm in B, respectively. These shift differences are large compared to the uncertainty in the theoretical calculation and might be used to identify the isomeric structures.
References and citations.
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838.DOI:10.1021/jo00356a016
- ↑ S. Leleu, C.; Papamicael, F. Marsais, G. Dupas, V.; Levacher, Vincent. Tetrahedron: Asymmetry, 2004, 15, 3919-3928.DOI:10.1016/j.tetasy.2004.11.004
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters,DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- ↑ B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447.DOI:10.1039/P29920000447
- ↑ Mamedov, V. A.; Levin, Ya. A. Khimiya Heterocycl. Soed. (Rus) 1996, 7, 1005.
- ↑ A. Balandina et al. / Tetrahedron Letters 45 (2004) 4003–4007