Rep:Module1:AYKLeung
Chemistry Computational Lab (Yr3)
Module 1: (organic) Molecular Mechanics (Structure and Spectroscopy)
In this exercise ChemBioDraw Ultra 12.0 and Gaussview 3 were extensively used for 3D molecular simulation.
The Hydrogenation of Cyclopentadiene Dimer
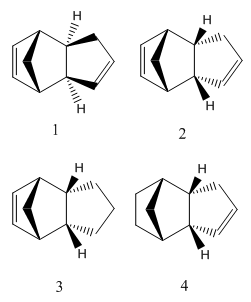
Two 1, 3-cyclopentadiene molecules would react through a Diels-Alder cycloaddition mechanism, as shown below. Theoretically 2 molecules would be formed, molecule 1, which is exo, and molecule 2, endo.
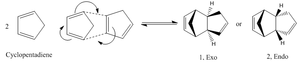
Repeated simulations were carried using ChemBioDraw Ultra 12.0, energies obtained for molecules 1 & 2 were 31.8809 kcal/mol and 34.0037 kcal/mol respectively. With the lower energy, Molecule 1 (exo) is the thermodynamically more favourable product of the reaction.
| Energy Term | Molecule 1 (Exo) /kcal/mol | Moelcule 2 (Endo) /kcal/mol |
|---|---|---|
| Stretch | 1.2820 | 1.2449 |
| Bond | 20.5771 | 20.8590 |
| Stretch-Bend | -0.8404 | -0.8362 |
| Torsion | 7.6451 | 9.5125 |
| Non-1,4 VDW | -1.4008 | -1.5624 |
| Dipole/Dipole | 0.3772 | 0.4493 |
| Total Energy | 31.8809 | 34.0037 |
However in a real-life situation it is the endo product (kinetically more stable) which is the major product. [1]
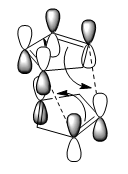
This could be explained by the Endo Addition Rule,image below shows the secondary orbital interactions, where the non-bonding carbons interact with each other, thus lowering the activation energy of the reaction.
When the endo product (2) is hydrolysed, 2 isomers were formed, molecules 3 & 4. Table 2 below shows all the energy terms of the isomers. It is shown that molecule 4 (31.1678kcal/mol) has a lower energy than molecule 3 (35.9285kcal/mol), which is expected as molecule 3 has a more hindered structure in terms of electronic density. If we look more closely to the individual energy terms, the largest contributor to the energy difference is by the "bend" energy term. This could be explained by the high strain at the base of the bridging carbons (see images below), the higher the strain the higher the energy of the compound. Therefore molecule 4 should be the major product of the reaction.

| Energy Term | Molecule 3 /kcal/mol | Moelcule 4 /kcal/mol |
|---|---|---|
| Stretch | 1.2348 | 1.1006 |
| Bend | 18.9227 | 14.5093 |
| Stretch-Bend | -0.7610 | -0.5459 |
| Torsion | 12.1538 | 12.5091 |
| Non-1,4 VDW | -1.5193 | -1.0495 |
| Dipole/Dipole | 0.1631 | 0.1408 |
| Total Energy | 35.9285 | 31.1678 |
Stereochemistry of Nucleophilic additions to a Pyridinium ring (NAD+ analogue)
There are two set of molecules to be simulated in this part.
a) Prolinol (molecule 5) reacting with methyl magnesium iodide alkylating the pyridine ring at the 4-position, forming product (molecule 6)[2]
A grignard reagent was used to methylate the prolinol. Magnesium is not registered as an atom in ChemBio3D and so it was not included in the reaction to prevent confusion during the optimisation. If included in the calculations an error bar would come up and say "No atom type was assigned to the selected atom!". A stable six-membered ring transition state was formed due to the electropositive magnesium and the electronegative oxygen. Measuring the dihedral angle between the oxygen and the carbon at the 4 position could be used as a tool to compare with the total energy of the molecule.
| Energy Terms | Conformer a (kcal/mol) | Conformer b (kcal/mol) | Conformer c (kcal/mol) |
|---|---|---|---|
| Stretch | 2.0547 | 23.2464 | 14.7186 |
| Bend | 14.2367 | 309.2128 | 133.3388 |
| Stretch-Bend | 0.1632 | -5.5241 | -3.6789 |
| Torsion | 6.1785 | 9.9505 | 11.4011 |
| Non-1,3 VDW | -0.5413 | 4.8365 | 2.0270 |
| 1,4 VDW | 16.7176 | 39.4210 | 34.3997 |
| Charge/Dipole | 9.7471 | 10.2393 | 10.8187 |
| Dipole/Dipole | -3.9448 | -1.7483 | -5.2170 |
| Total energy | 44.6117 | 389.6341 | 198.8080 |
| Dihedral angle (o) | 12.2524 | 177.1757 | -6.4102 |

Conformer a has the lowest energy. It is observed that the main contributor among the energy terms is the "Bend" term. With a large angle (e.g. conformer b, 177o), the "Bend" term is massive (309kcal/mol).
The methylated product has a lower energy than the NAD+ reactant.
| Energy Terms | NAD+ (kcal/mol) | methylated product (kcal/mol) |
|---|---|---|
| Stretch | 2.0547 | 2.2665 |
| Bond | 14.2367 | 16.4232 |
| Stretch-Bend | 0.1632 | 0.3757 |
| Torsion | 6.1785 | 6.7434 |
| Non-1,4 VDW | -0.5413 | -1.5624 |
| 1,4 VDW | 16.7176 | 17.7504 |
| Charge/Dipole | 9.7471 | / |
| Dipole/Dipole | -3.9448 | -4.2534 |
| Total Energy | 44.6117 | 39.1444 |
Main energy difference between them is the charge dipole term due to the positive charge on the nitrogen atom in the NAD+ molecule.
b) The pyridinium ring of molecule 7 reacted with aniline to form molecule 8, adding an NHPhenyl group to the 4-position of the pyridine ring. This reaction is similar to the one above, which produces a stereo-specific product. [3]

The position of the carbonyl group determines which side the aniline attacks from because of the main electronic repulsion between the lone pair at the nitrogen of the aniline and the oxygen atom and also the steric hindrance between the phenyl group and the oxygen atom. In order to find the most stable conformer of molecule 7 the energies and dihedral angles of several conformers were investigated.
| Energy Terms | Conformer a (kcal/mol) | Conformer b (kcal/mol) | Conformer c (kcal/mol) |
|---|---|---|---|
| Stretch | 3.8637 | 4.1180 | 5.6855 |
| Bend | 11.4808 | 13.4132 | 17.1402 |
| Stretch-Bend | 0.3953 | 0.4447 | 0.5050 |
| Torsion | 10.2675 | 9.9805 | 18.7684 |
| Non-1,3 VDW | 3.9413 | 4.8287 | 6.8182 |
| 1,4 VDW | 29.2398 | 29.2288 | 30.0828 |
| Charge/Dipole | 9.0276 | 9.2580 | 7.6538 |
| Dipole/Dipole | -4.8834 | -4.8696 | -4.8987 |
| Total energy | 63.4425 | 66.4024 | 81.7552 |
| Dihedral angle (o) | -21.3929 | -18.1639 | -31.6692 |
The optimal dihedral angle was found to be -21o. With a negative angle it means that the carbonyl oxygen is pointing below the ring, thus suggesting a stereo-specific attack of the aniline from the top.
Stereochemistry and Reactivity of an Intermediate in the synthesis of Taxol
The intermediates of Taxol (molecules 9 & 10, as demonstrated below) exist as atropisomers. Also existing in chair and twisted boat conformations, there is a total of 4 isomers to be looked at.

| Energy Terms (in MM2) | Molecule 9 as twisted boat (kcal/mol) | Molecule 9 as chair (kcal/mol) | Molecule 10 as twisted boat (kcal/mol) | Molecule 10 as chair |
|---|---|---|---|---|
| Stretch | 2.8258 | 2.8201 | 2.4256 | 2.4929 |
| Bend | 16.4410 | 16.8250 | 11.9996 | 10.9433 |
| Stretch-Bend | 0.4580 | 0.3696 | 0.2037 | 0.3001 |
| Torsion | 21.3785 | 20.7889 | 16.3124 | 17.2833 |
| Non-1,4 VDW | -0.9086 | -0.2776 | -1.3151 | -1.7218 |
| 1,4 VDW | 14.0441 | 14.1993 | 12.1331 | 12.4238 |
| Dipole/Dipole | 0.1368 | 0.2776 | 0.2599 | 0.1433 |
| Total energy | 55.3756 | 55.0747 | 42.0193 | 41.8649 |
| MMFF94 | 76.3397 | 76.1923 | 60.1686 | 60.0065 |
This shows that molecule 10 is a thermodynamically more stable compound than molecule 9 is. Also both compounds prefer twisted boat over a chair conformation. The "bend" term contributes most to the difference in energies of the isomers, due to the strain of the bonds at the chair conformation. The MMFF94 minimised energies give a slightly higher value that that of the MM2 method, but agrees with the trend that the MM2 energies gives. Note that both molecules 9 and 10 are hyperstable alkenes (hyperstable olefins), which explains why they react more slowly than other alkenes. This double bond contained in a 9-membered ring has a comparatively less strain than than the parent alkane. The cage structure also provides protection. Therefore this alkene bond is less likely to be hydrogenated and is thermodynamically more stable than other functional isomers.
Semi-Empirical Molecular Modelling Theory
In this part the Mechanical Molecular Modelling Technique is investigated, e.g. the Diels-Alder reaction where secondary orbital interactions were involved and caused a break down in the model. In this section, similar reactivity will be considered, and how the electrons influence bonds and spectroscopic properties.
Regioselective addition of Dichlorocarbene

Firstly a MM2 method was run to minimise the energy and clean up the geometry before applying an electronic method. Next the MOPAC/PM6 was run to approximate the valence-election wave functions, especially the HOMO as it is the most reactive towards electrophillic attacks. The molecular orbitals from HOMO-1 to LUMO+2 are shown below.
| HOMO-1 | HOMO | LUMO | LUMO+1 | LUMO+2 |
|---|---|---|---|---|
 |
 |
 |
 |
|
The HOMO and LUMO are the most important orbitals to be considered while looking at bond breaking/forming. It was found that it was a highly selective addition at the double bond syn to the C-Cl bond. This could be explained by the rich electron cloud surrounding the double bond on the Cl side of the molecule as demonstrated by the HOMO, and is therefore more nucleophilic. The rich electron density could be explained by the antiperiplanar overlap between the C-Cl σ* (LUMO+1) and the π bond (HOMO-1), thus creating a large stabilising effect on the other alkene orbitals.
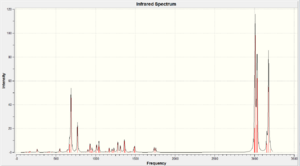
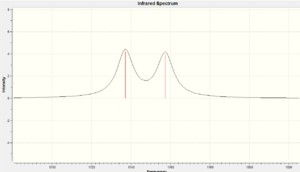
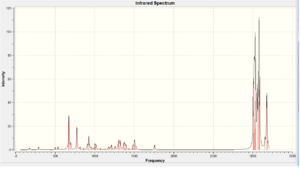
Vibrational Frequencies
The vibrational frequencies are calculated using the Gaussian interface approach B3LYP/6-31G(d,p). The following two molecules are investigated.

 |
 |

|
|---|---|---|
 |
|
 |
 |
|
|---|---|---|
 |

|
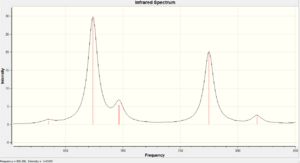
| Vibration Type (cm-1) | Diene (molecule 12) | Monoene (molecule 13) |
|---|---|---|
| C=C | 1737.09/1757.36 | 1758.05 |
| C-Cl | 770.902 | 784.942 |
There is a considerably large difference between the Diene C-Cl bond energy and the Monoene C-Cl bond energy (14.04cm-1). This is due to the orbital interactions between the HOMO-1 and LUMO+1. They have similar values at the C=C bond vibration and the other C=C is about 20cm-1 lower than the other one. This could be due to interactions between the HOMO and LUMO+2 where they affect the bond strength.
Structure-Based Mini Project
Investigations on Diels-Alder reaction of phospholes with olefinic dienophiles has not been extensively studied, but only the cycloaddition of 3,4-dimethyl-1-phenylphosphole with N-phenylmaleimide (NPMI) and fumaronitrile has been looked at (see image below). This reaction produces an endo product, as predicted in part 1 of this whole exercise. Due to the high sterics of the P-aryl group, the phosphorus pyramin at the phospholes was planarised to allow some electron delocalisation.
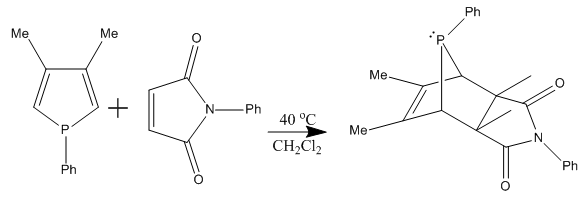
This has lead to further investigations in the following mini project.
Two isomers are formed in the reaction, molecules A & B. The pentadiene reacts with the double bond at the NPMI, forming 2 isomers. As explained above, how the P-aryl group is facing would affect the amount of electron delocalisation spread out throughout the molecule.

The carbons are labelled in such a way shown in the following image.
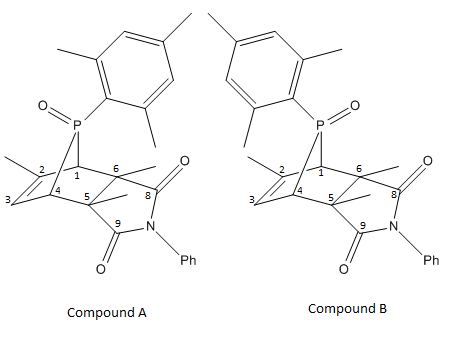
| Compound A | Compound B |
|---|---|
 |

|
 |

|
 |
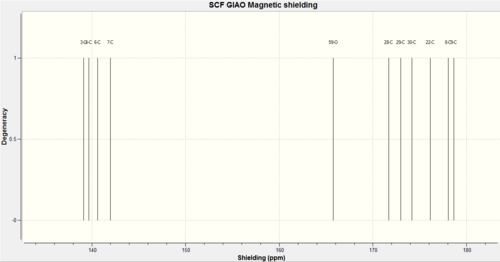
|
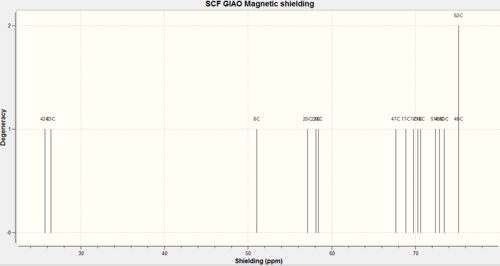
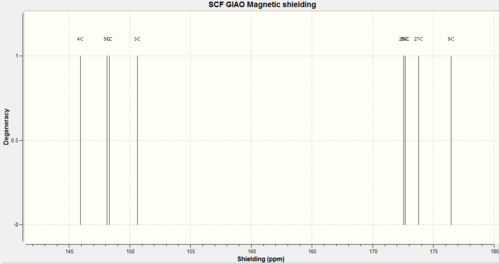
Carbon labels were different between Gaussview and the literature values, therefore carbon labels at the reference values can not be considered the same as the values in the mini project.
| Carbon label (shift values) | Compound A Experimental Value | Compound A Calculated Value | Compound B Experimental Value | Compound B Calculated Value |
|---|---|---|---|---|
| 1 | 50.4 | 51.1 | ||
| 2 | 142.0 | 141.7 | ||
| 3 | 122.5 | 122.2 | ||
| 4 | 47.0 | 47.7 | ||
| 5 | 42.4 | 42.5 | ||
| 6 | 41.0 | 41.1 | ||
| 8 | 175.0 | 174.6 | ||
| 9 | 175.4 | 175.9 |
References
- ↑ W.C. Herndon, C.R. Grayson, J.M. Manion, J. Am. Chem. Soc.., 2002, 124, 1130 DOI:10.1021/jo01278a003
- ↑ DOI| 10.1021/jo00356a016
- ↑ doi|10.1016/j.tetasy.2004.11.004
- ↑ doi:10.1016/S0040-4039(00)92617-0
- ↑ http://hdl.handle.net/10042/to-5693
- ↑ http://hdl.handle.net/10042/to-5694
